Delftia is an aerobic, Gram-negative, oxidase-positive, non-glucose-fermenting bacillus. Delftia species are ubiquitous in water and soil.1 However, they are rarely associated with human infections. To date, four Delftia species (D. acidovorans, D. tsuruhatensis, D. lacustris, and D. litopenaei) have been described. D. acidovorans (formerly known as Comamonas acidovorans) and D. tsuruhatensis have been reported as causes of human infections such as catheter-related bacteremia, pneumonia, empyema, peritonitis in a patient receiving peritoneal dialysis, urinary tract infections, and ocular infections.2 However, there has been no report of ocular infection by D. lacustris. Herein, we present a patient with keratitis and probable endophthalmitis caused by D. lacustris.
A-70-year-old male farmer visited the hospital with a complaint of painful foreign body sensation and epiphora in his left eye. His symptoms developed following non-penetrating eye trauma from a tree branch while picking red peppers two weeks earlier. He had been taking anti-hypertensive and anti-diabetic medications for over 30 years, but was otherwise in good health. On examination, visual acuity without correction was 20/25 in his right eye and he could count fingers at 30cm using his left eye. The left eye demonstrated a corneal infiltrate and an approximately 1mm corneal epithelial defect. The right eye was clear. Cultures of corneal scrapings and eye discharge grew Gram-negative bacilli. Isolates were identified as D. acidovorans by Vitek 2 system (bioMérieux Inc., Durham, NC, USA). Because isolation of D. acidovorans from the eye is rare, we sent the isolate to the Infectious Disease Research Institute (IDRI) at the Asia Pacific Foundation for Infectious Diseases (APFID) for further testing. The 16S rRNA gene sequence (1276bp) that was identified was compared using BLAST searches of the GenBank and EzTaxon servers (http://www.ezbiocloud.net/eztaxon). The sequence was 99.92% identical to that of D. lacustris (GenBank accession number EU888308). The second and the third closest matches were D. tsuruhatensis and D. acidovorans with 99.84% and 98.51% homology, respectively (accession numbers AB075017 and AF078774). Since our strain could utilize D-mannitol and D-malic acid (API 20 NE, bioMérieux Inc.) for growth, it was determined to be D. lacustis. The isolates were susceptible to aztreonam, cefepime, ceftazidime, piperacillin/tazobactam, and carbapenems, but resistant to aminoglycosides according to the Vitek system (bioMérieux Inc.) using 2011 Clinical Laboratory Standards Institute criteria for Pseudomonas (Table 1). The patient was initially treated with fortified topical ofloxacin, voriconazole, and gentamicin. Antibiotics were switched to topical ciprofloxacin and systemic ceftazidime after isolation of Delftia. However, the patient did not respond to therapy, and two months later underwent evisceration.
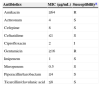
Antimicrobial susceptibility profiles for Delftia lacustirs.
| Antibiotics | MIC (μg/mL) | Susceptibilitya |
|---|---|---|
| Amikacin | ≥64 | R |
| Aztreonam | 4 | S |
| Cefepime | 8 | S |
| Ceftazidime | ≤1 | S |
| Ciprofloxacin | 2 | I |
| Gentamicin | ≥16 | R |
| Imipenem | 1 | S |
| Meropenem | 0.5 | S |
| Piperacillin/tazobactam | ≤4 | S |
| Ticarcillin/clavulanic acid | ≤8 | S |
MIC, minimum inhibitory concentration; S, susceptible; R, resistant.
D. lacustris was first described in 2009 in freshwater in Denmark.3D. lacustris and D. tsuruhatensis have 99.9% nucleotide similarity in the 16S rRNA gene sequence, as shown in this report. They can be differentiated based on the use of certain carbon sources for growth, such as d-mannitol and d-malic acid, as well as chitinase activity.3 In this study, the isolate was determined to be D. lacustris as d-mannitol and d-malate were utilized for growth in the API 20 NE system (bioMérieux). Shin et al. reported four possible human infections caused by D. lacustris. However, all of these were considered to be contaminants because only one bottle out of two sets of blood cultures grew Delftia, and some patients recovered without antibiotic therapy.4 Very recently, we described a true bloodstream infection by D. lacustris,2 which was initially identified as D. acidovorans by Vitek 2 system, as in this report. All four D. lacustris infections reported by Shin et al. were also originally identified as D. acidovorans by Vitek 2.4 This indicates that infections due to D. lacustris may be more common than previously thought due to misidentification by commercial systems.
According to species-independent clinical breakpoints provided by the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2014), our isolate was susceptible to aztreonam, ceftazidime, cefotaxime, piperacillin–tazobactam, ticarcillin–clavulanate, and carbapenems, but resistant to all aminoglycosides tested and ciprofloxacin. Delftia is generally considered resistant to aminoglycosides.5 Ceftriaxone and cefotaxime may be effective for the treatment of Delftia infections because endocarditis caused by D. acidovorans has been successfully controlled using ceftriaxone alone.1 Although the minimum inhibitory concentrations (MICs) of trimethoprim–sulfamethoxazole, minocycline, and tigecycline were very low, we do not know the clinical implications of this because there are no antibiotic susceptibility criteria or guidelines for Delftia species.
This is the first report of ocular infection with D. lacustris accompanied by significant complications. Because commercial systems can misidentify Delftia species, molecular methods such as 16S rRNA gene sequencing may be required. Further clinical investigation of D. lacustris is necessary to determine optimal therapy for this unusual pathogen.
Conflicts of interestThe authors declare no conflicts of interest.