Mycobacterium neoaurum is a rare cause of bacteremia, and infection usually occurs in an immunocompromised host in the setting of an indwelling catheter. Prosthetic valve endocarditis due to non-tuberculous mycobacteria typically carries a dismal prognosis; we report a case of M. neoaurum prosthetic valve endocarditis with favorable response to antimicrobial therapy without surgical intervention.
A 30-year old Caucasian male presented to the emergency department with shortness of breath and palpitations of two weeks duration associated with fever and chills. His past medical history was significant for multiple episodes of MRSA (methicillin resistant Staphylococcus aureus) endocarditis in the setting of intravenous drug use, for which he had undergone bovine mitral valve replacement a year prior. He admitted to continued intravenous drug use. His vital signs on admission were temperature of 101.4°F, pulse rate of 106 per minute, blood pressure of 98/58mmHg and respiratory rate of 20 per minute. A grade 3/6 holosystolic murmur was heard best at the mitral area and radiated to the axilla. The lower extremities were warm to touch. Distal pulses were intact and symmetric. A detailed neurological exam was unremarkable. Admission laboratory tests showed a WBC count of 16.8K/mm3 with neutrophil predominance (84%). Serum electrolytes, blood urea nitrogen, creatinine, creatine kinase (CK) and liver function tests were within the normal range. Chest X-ray showed no abnormality, and electrocardiogram showed normal sinus rhythm with mild left atrial enlargement.
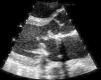
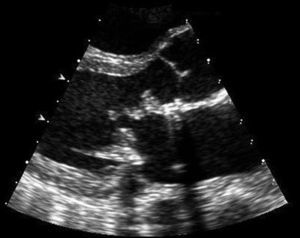
Blood cultures were drawn and the patient was started on empiric antibiotic therapy with vancomycin, ceftriaxone and rifampin for provisional diagnosis of prosthetic valve endocarditis. A transthoracic echocardiogram revealed extensive vegetations on the prosthetic mitral valve with mild obstruction to mitral inflow and a normal ejection fraction (Fig. 1). The Gram stain of the blood smear was reported as clusters of rods with Gram positive characteristics and the acid fast stain (Kinyoun) was positive for acid-fast bacteria. Three blood culture bottles (aerobic) grew yellow shiny colonies suggestive of Mycobacteria in 10 days. The organism was identified as Mycobacterium neoaurum using high-pressure liquid chromatography. The patient was treated with intravenous tobramycin, oral azithromycin and oral moxifloxacin based on susceptibility testing results. On day 10 of his hospital stay he developed severe abdominal pain. CT scan of the abdomen revealed a splenic and left renal infarct. Magnetic Resonance Imaging (MRI) of brain revealed one centimeter brain abscess. Surgical intervention was discouraged given his ongoing drug use. The patient's condition improved remarkably within the next few days. His repeat blood cultures were negative on day 4 and his symptoms resolved. Although it was advised that he continue the same antimicrobial regimen for an extended period of time, he was unwilling to do so. He was finally discharged on day 39 on a regimen of oral azithromycin, ethambutol and moxifloxacin. The patient did not follow up with his physicians, and he was readmitted a year later for prosthetic valve endocarditis due to coagulase negative staphylococcal species. His AFB cultures drawn at this time were negative. He responded to intravenous antibiotics and surgical management was once again not pursued given his ongoing intravenous drug use.
DiscussionInfective endocarditis due to mycobacterium species is an unusual but established clinical entity. Nontuberculous mycobacteria (NTM) have been reported more frequently as a cause of infective endocarditis than Mycobacterium tuberculosis, especially of prosthetic valves.1,2 The commonly identified NTM species causing endocarditis have included the following rapidly growing mycobacteria: Mycobacterium fortuitum, Mycobacterium abscessus, and Mycobacterium chelonae.2,3 NTM PVE has been described in both mechanical and biologic valvular prostheses. M. neoaurum has been reported rarely as a cause of bloodstream infections in immunocompromised hosts, such as patients with malignancies or transplant recipients.4M. neoaurum has also been implicated in pulmonary infections,5 meningoencephalitis,6 urinary tract infection,7 and catheter related infections.4,8 van Duin et al. reported the first case of PVE due to M. neoaurum with good outcome in 2010.9 In our patient, the source of M. neoaurum was likely contamination during illicit intravenous drug injection.
M. neoaurum can be detected on routine aerobic blood cultures and is a rapidly growing organism on Lowenstein–Jensen agar. The organism grows at temperatures between 25 and 35°C, usually within five days. M. neoaurum colonies also have a characteristic yellowish-orange smooth appearance that is distinct from the colony characteristics of non-chromogenic mycobacterium species. In many of the reported cases of endocarditis due to NTM the blood cultures were negative and the identification of the NTM was done by culturing the removed prosthetic valve, or by histopathology analysis in conjunction with molecular assays. The advanced methods for organism identification include high-pressure liquid chromatography and genotypic methods like sequencing of unique 16S rRNA.4 The 16S rRNA utilizes the hypervariable nucleotide sequences that lend species-specific variability within members of the same genus. Therefore, if blood cultures are negative in a patient suspected of having PVE, definitive diagnosis can be made by removal of the infected prosthetic valve and performance of the aforementioned studies. In an immunocompromised patient, however, unexplained fever and symptoms of infection along with isolation of M. neoaurum, particularly in multiple specimens, should be considered highly suggestive of infection with this organism.
Susceptibility testing of rapidly growing mycobacteria has several limitations: it is not standardized; results are often delayed well into empiric therapy, and most importantly there is a lack of data linking clinical response to in vitro test results. Presently, the minimum recommendations for antibiotic susceptibility testing of rapidly growing NTM include clarithromycin, amikacin, cefoxitin, imipenem (the carbapenem preferred over meropenem and ertapenem), tobramycin, doxycycline, linezolid, ciprofloxacin, and sulfonamides.10M. neoaurum displays excellent susceptibility to ciprofloxacin while resistance to it is now widely encountered amongst other rapidly growing NTM. A recent study, however, did demonstrate high resistance for M. neoaurum to clarithromycin.11 Combination antimicrobial therapy including macrolides, fluoroquinolones and aminoglycosides is a better therapeutic approach than monotherapy owing to diagnostic delay in organism identification and varying susceptibility and resistance patterns for different mycobacteria species. Endocarditis due to NTM, particularly involving the prosthetic valves, carries a dismal prognosis and requires prolonged antibiotic therapy (ranging between 47 days and 187 days).3 Previously reported catheter related M. neoaurum infections required a minimum of three weeks of antimicrobial therapy, with recovery augmented by removal of the infected catheter.4 While prosthesis removal would have been ideal, the good therapeutic response in our patient could be due to the indolent nature and low pathogenicity of M. neoaurum and the excellent susceptibility profile it displays in contrast to other NTM.12 This is further corroborated by the remarkable response to therapy for the patient reported by van Duin et al. and for majority of the patients who had infections of indwelling catheters due to M. neoaurum.4,9 Since there is lack of considerable treatment experience for M. neoaurum endocarditis involving the prosthetic valves, we recommend several weeks of combination antimicrobial therapy and removal of the infected prosthesis when possible.
In conclusion, NTM PVE is a well-defined clinical entity that should be considered in patients with prosthetic valves who present with symptoms of endocarditis with negative blood cultures. M. neoaurum is a rapidly growing NTM that can cause infective endocarditis in immunocompromised individuals. NTM PVE due to M. neoaurum, in contrast to PVE due to other NTM, may be amenable to long term antibiotic therapy, especially if combined with prosthesis removal. In the absence of definitive regimens and substantial experience, physicians can rely on antibiotic susceptibility data and previous case reports to guide their antibiotic choice and duration of therapy. In general, however, endocarditis secondary to NTM, particularly involving the prosthetic valves, carries a poor prognosis.
Conflict of interestThe authors declare no conflicts of interest.