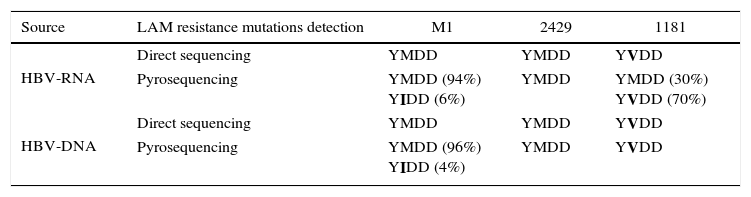
Hepatitis B virus (HBV) is a unique DNA virus that replicates via pre-genomic RNA.1 According to current estimates, approximately 4 million individuals living with HIV worldwide have chronic hepatitis B (CHB).2 For the treatment of HBV/HIV co-infected patients, several guidelines recommend the use of nucleos(t)ide analogues (NA), such as a combined lamivudine (LAM) and tenofovir (TDF) therapy.3 During long-term NA therapy, drug-resistant mutations emerge, resulting in an increase of HBV-DNA expression. During LAM therapy, the most classical mutations are observed in the tyrosine-methionine-aspartate-aspartate (YMDD) motif of the HBV-DNA polymerase at reverse transcriptase domain.3 Low HBV-DNA load in serum may hamper the detection of drug resistance mutations in HBV/HIV co-infected patients. Serum HBV-RNA can be found during LAM therapy and it can be detected when HBV-DNA is undetectable. The objectives of this study were to evaluate HBV-RNA detection in serum of HBV/HIV co-infected patients under antiviral treatment and to detect the early emergence of LAM resistance mutations by pyrosequencing. Ten CHB male patients were enrolled in this study and divided into three groups: I- six HBV/HIV co-infected patients treated with LAM and TDF for one year (M1 and 2429); three and five years (1181 and 3013), and ten years (3107 and 1452); II- two HBV monoinfected naïve patients; and III- two HBV/HIV co-infected naïve patients. Serum samples collected from enrolled patients who provided written informed consent were obtained and stored at -80°C. This study was approved by the ethical committee of Oswaldo Cruz Foundation with ref. no. 575/10. Serum samples were tested for HBsAg and HBeAg by a commercial immunoassay BioELISA HBsAg colour, Biokit (Barcelona, Spain) and DiaSorin ETI-EBK PLUS (Vercelli, Italy) respectively, according to the manufacturer's instructions. Total nucleic acid was extracted using the High Pure Viral Nucleic Acid kit (Roche, Konzern-Hauptsitz, Switzerland) that allows simultaneous detection of DNA and RNA, according to the manufacturer's instructions. For HBV-DNA detection, small S region was amplified by a semi-nested PCR assay that amplifies HBV genotypes A-H.4 For HBV-RNA detection, a RT-PCR using the Superscript III one-step RT-PCR kit (Invitrogen, Carlsbad, CA) was carried out, amplifying the preS/S region. cDNA synthesis was performed by real-time PCR using M-MLV RT (Promega Corp., Madison, WI, USA) according to the manufacturer's instructions. Phylogenetic analysis identified HBV genotypes A and D. Pyrosequencing of a fragment of the HBV polymerase gene, containing the YMDD motif, was performed according to a previously described procedure5 using PyroMark Q96 ID (QIAGEN Valencia, CA, USA). This pyrosequencing assay was accurate for detecting mutations in minor subpopulations as low as 5% of the total. HBV-DNA and HBV-RNA (cDNA) viral loads were determined by real-time PCR. HBV-DNA and/or RNA were detected in serum samples from 4/6 HBV/HIV co-infected patients under NA therapy. HBV-RNA load was much higher than the HBV-DNA. M204V/I resistance mutation was investigated in HBV-DNA and/or HBV-RNA and mixtures of wild type and mutant populations were detected (Table 1). HBV-RNA can be a useful tool for monitoring the emergence of drug resistance mutations in HBV/HIV co-infected patients under antiviral treatment.
Detection of LAM resistance mutations by direct sequencing and pyrosequencing.
| Source | LAM resistance mutations detection | M1 | 2429 | 1181 |
|---|---|---|---|---|
| HBV-RNA | Direct sequencing | YMDD | YMDD | YVDD |
| Pyrosequencing | YMDD (94%) YIDD (6%) | YMDD | YMDD (30%) YVDD (70%) | |
| HBV-DNA | Direct sequencing | YMDD | YMDD | YVDD |
| Pyrosequencing | YMDD (96%) YIDD (4%) | YMDD | YVDD |
This work was supported by Foundation for Research Support of Rio de Janeiro Carlos Chagas Filho (FAPERJ) and National Council for Scientific and Technological Development (CNPq).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like thank to Katharina Röltgen and Elza Carmem Cerqueira de Almeida for fruitful discussions and Claudia Rosa Lucio Kamel for the English revision.