Invasive fungal infections (IFIs) affect >1.5 million people per year. Nevertheless, IFIs are usually neglected and underdiagnosed. IFIs should be considered as a public-health problem and major actions should be taken to tackle them and their associated costs. Aim To report the incidence of IFIs in four Mexican hospitals, to describe the economic cost associated with IFIs therapy and the impact of adverse events such as acute kidney injury (AKI), liver damage (LD), and ICU stay.
MethodsThis was a retrospective, transversal study carried-out in four Mexican hospitals. All IFIs occurring during 2016 were included. Incidence rates and estimation of antifungal therapy's expenditure for one year were calculated. Adjustments for costs of AKI were done. An analysis of factors associated with death, AKI, and LD was performed.
ResultsTwo-hundred thirty-eight cases were included. Among all cases, AKI was diagnosed in 16%, LD in 25%, 35% required ICU stay, with a 23% overall mortality rate. AKI and LD showed higher mortality rates (39% vs 9% and 44% vs 18%, respectively, p<0.0001). The overall incidence of IFIs was 4.8 cases (95% CI=0.72–8.92) per 1000 discharges and 0.7 cases (95% CI=0.03–1.16) per 1000 patients-days. Invasive candidiasis showed the highest incidence rate (1.93 per 1000 discharges, 95% CI=−1.01 to 2.84), followed by endemic IFIs (1.53 per 1000 discharges 95% CI=−3.36 to 6.4) and IA (1.25 per 1000 discharges, 95% CI=−0.90 to 3.45). AKI increased the cost of antifungal therapy 4.3-fold. The total expenditure in antifungal therapy for all IFIs, adjusting for AKI, was $233,435,536 USD (95% CI $6,224,993 to $773,810,330).
ConclusionsIFIs are as frequent as HIV asymptomatic infection and tuberculosis. Costs estimations allow to assess cost-avoidance strategies to increase targeted driven therapy and decrease adverse events and their costs.
Invasive fungal infections (IFIs) affect more than 1.5 million people per year, which is similar to the burden of infections due to tuberculosis and 3-fold more than malaria.1 Despite this, IFIs are usually neglected and underdiagnosed especially in low- and middle-income countries, including those with endemic mycoses. The principal reasons for misdiagnosing are the absence of diagnostic tools plus insufficient training of health-care staff.2,3
The lack of diagnostic tests based on culture and non-culture techniques, can lead to overuse of empirical therapy, mainly for individuals with hematological diseases and critically ill patients.4,5 Previous European reports have shown that antifungals are initiated as empirical therapy in more than 60% of the cases, which could lead to toxic adverse effects associated to unnecessary therapy and to higher costs.6,7
IFIs should be considered as a public health problem, therefore a number of major actions should be taken to address these infections and their associated costs. These actions include insurance coverage of affordable diagnostic tests, improvement of mycology expertise across low- and middle-income countries’ diagnostic laboratories, safeguarding the distribution of essential antifungal agents, establishment of an official reporting and surveillance system for these infections.3,8 These measures are also key elements of an effective antifungal stewardship program.9
In this study, we report the incidence rates of IFIs in four Mexican hospitals, describe the economic cost associated with IFIs therapy and the impact of adverse events such as acute kidney injury, liver damage, and ICU stay.
MethodsSettingMexico has a divided health system where public health care is furthered divided into people with and without social insurance. Uninsured Mexicans’ health expenditure can be out of pocket or covered by the Seguro Popular (SP). The SP consists of well defined benefit packages and medicines that provides coverage only for certain services such as preventive medicine and primary care, national vaccination program, HIV infection care, and catastrophic medical expenditures associated with certain conditions such as critical care, neonatal care, congenital disorders, certain malignancies, hepatitis C infection, stem-cell, and solid-organ transplants (with different limitations regarding age).10
Participating centersFour hospitals located in different states around the country that provide medical attention to patients without social security were included.
In all the hospitals, patients’ socioeconomic level (SEL) was evaluated upon admission and many of the expenses are charged according to the socioeconomic level.
The National Cancer Institute (INCan) of Mexico City is a 150-bed tertiary care center focused on the treatment of adult oncological patients who are referred mostly from the central part of the country for medical attention. For diseases not included in the SP, hospital expenses are paid according to the SEL assigned, but medication costs are absorbed 100% by the patient, regardless of SEL.
The Regional High Specialty Hospital of Oaxaca (HRAEO) is a 100-bed tertiary care center located in Oaxaca City, Southwest Mexico. It provides service only to adults, without gynecology and obstetrics department. The patients pay the hospital length of stay (LOS) and procedures according to the assigned SEL, the rest is absorbed by the hospital. The costs of drugs included in the basic hospital chart, are 100% absorbed by the hospital.
The University Hospital “Dr. José Eleuterio González” (HUNL) is a 667-bed general hospital in Nuevo León, Northeast Mexico. The hospital is part of the Autonomous University of Nuevo León, providing total hospital attention to around 50% of the patients without medical insurance in the entire state. Patients treated in the hospital are predominantly from Nuevo León and surrounding Northeast states. All expenses are paid according to the SEL.
The Regional High Specialty Hospital of El Bajío (HRAEB), in León Guanajuato, North Central Mexico, is a 184-bed tertiary care center, specialized in adult oncological patients as well as hematopoietic, renal and hepatic transplantation, and highly specialized surgery. Patients are referred from the middle and east part of the country. Almost 100% of them have SP, and more than half of them are covered by the catastrophic funding; total expenses of hospitalization and medication costs are absorbed 100% by federal government. In all the hospitals, patients with diseases not covered by SP or catastrophic diseases pay hospital expenses according to the SEL, and purchase medications not provided by the hospital at full cost.
Study design and data collectionThis was a retrospective and cross-sectional study approved by each local institutional review board (IRB) with the following register numbers: HUNL IF17-00001, HRAEB CEI-08-17, INCAN CI/381/17 and HRAEO-CIC 003-17.
All IFIs occurring during 2016 were identified using the databases of each participating hospital. Clinical data was recovered from medical records. This included demographic information, previous comorbidities, type of IFI, antifungal type used, duration, and indication, as well as, outcomes such as development of acute kidney injury (AKI), liver damage (LD), ICU admission, hospital LOS, and mortality.
The following definitions were used:
- •
AKI was defined as a ≥two-fold increase in serum creatinine compared to baseline level and/or presence of hypokalemia, or hypomagnesaemia during antifungal therapy.
- •
LD was defined as a ≥two-fold increase in AST/ALT or total bilirubin compared to baseline level during antifungal therapy.
- •
IFIs were classified according EORTC/MSG criteria.11 Possible IFIs were categorized as possible IFIs in neutropenic fever (NF) individuals or possible IFIs in non-NF individuals.
Data regarding costs of antifungal drugs, hospital, and ICU stay were provided by each participant hospital for each patient. In Mexico, hospitals buy certain authorized antifungal drugs at pre-established prices during public tenders (Supplementary material, Table S1). Antifungals such as liposomal amphotericin B (LAmB) and posaconazole are usually not part of the hospitals’ stock due to their high prices. Hence, LAmB should usually be purchased by the patients at drug stores or via a non-governmental organizations (NGOs). Estimate expenses for antifungals were calculated based on the prices paid by each hospital (Table S1).
All identified IFI-cases without clinical data available were not included for either clinical nor cost analysis, however they were included for incidence rates calculation.
Incidence rates estimationOnly proven and probable IFIs were used to estimate incidence rates. These were calculated by type and an overall incidence rate for all types of IFIs. The incidence rates were estimated using the number of discharges and patients-days during 2016.
Cost of IFIs analysis per year in MexicoAn estimation of the total expenditure in antifungal therapy during one year in Mexico was performed based on the number of hospital discharges during 2015 (last information available). This information, was gathered from the Organization for Economic Cooperation and Development (OECD) webpage, in December 2017.12 The rest of the estimations was calculated with data obtained in this study, such as incidence rate for proven/probable IFIs, mean cost of antifungal therapy, mean days of treatment. Costs were adjusted by the expected proportion of AKI and increasing cost of antifungal therapy due to AKI.
Statistical analysisThis study data is presented as proportion, median and interquartile range (IQR) depending on the type of data. For categorical data comparisons, Pearson's chi-square or Fisher's exact test were used as appropriate and for ordinal and quantitative variables, Mann–Whitney or Kruskal–Wallis tests were used as indicated. After univariate analysis, those variables with a p-value ≤0.05 were included in a binary logistic regression multivariate analysis of factors associated with death, AKI and LD, and adjusted for sex and age. Statistical analysis and plot construction were performed using SPSS 24 (IBM Chicago, US) and Prism 7 (Graph Pad, California, US) software. Mean and 95% confidence intervals were estimated as required for the yearly expenditure analysis and incidence rates.
ResultsGeneral characteristics of the populationA total of 238 cases were included in the analysis. Most of the cases had a hematological malignancy (34%), followed by a solid tumor (16%), diabetes mellitus (10%) and HIV infection (8%) (Table 1). The main indication for antifungal therapy in this sample was empiric in 59% (140/238) of the cases, most of them to treat possible IFIs in non-neutropenic individuals (101/140, 72%); in the remaining 41% the indication was to treat proven or probable IFI (Table 1). Ninety-four percent of the individuals with a NF-possible IFI (37/39) had a hematological malignancy and/or solid tumor. In contrast, only 33% of the individuals with a non-NF possible IFI had either these comorbidities (Table S2).
General characteristics of the studied population in four Mexican hospitals.
| Characteristic | N=238 (%) |
|---|---|
| Age, yrs (median, IQR) | 37 (22–54) |
| Female sex | 105 (44) |
| Comorbidities | |
| Hematological malignancies | 80 (34) |
| Lymphocytic acute leukemia | 28/80 (35) |
| Myeloid acute leukemia | 23/80 (29) |
| Other | 29/80 (36) |
| Solid neoplasia | 38 (16) |
| Diabetes mellitus | 25 (10) |
| HIV infection | 20 (8) |
| Autoimmune disease | 13 (5.5) |
| Heart disease | 13 (5.5) |
| Renal transplant | 13 (5.5) |
| Other (hepatic cirrhosis or other gastrointestinal disease, and neurologic, genitourinary diseases) | 36 (15) |
| Primary indication for Antifungal therapy | |
| Non-NF possible IFI | 101 (42.5) |
| Proven/probable IFI | 98 (41) |
| Neutropenic fever possible IFI | 39 (16.5) |
| First antifungal used (before having the final diagnosis) | |
| Fluconazole | 85 (36) |
| Echinocandins (caspofungin, anidulafungin) | 68 (29) |
| AMBD | 38 (16) |
| Voriconazole | 19 (8) |
| Lipid formulation of AMB | 13 (5) |
| Other (posaconazole, itraconazole) | 12 (5) |
| Second antifungal used (N=80/235, 33%) (adjusted management) | |
| Fluconazole | 23 (29) |
| Voriconazole | 15 (19) |
| Caspofungin | 10 (13) |
| Itraconazole | 10 (13) |
| Lipid formulation of AMB | 8 (11) |
| AMBD | 7 (9) |
| Duration of antifungal therapy (days, median, IQR) | 10 (5–18) |
| Acute kidney injuryb | 39 (16) |
| Liver damage during antifungal treatment (LD)b | 58 (25) |
| Length of hospital stay (days, median, IQR) | 20 (12–31) |
| ICU stay | 83 (35) |
| Length of ICU stay (days, median, IQR) | 11 (5–19) |
| Mortality ratec | 54 (23) |
| Total cost of antifungal drug (US dollars, median, IQR)a | 232 (8–1044) |
| Total cost associated with hospital stay (US dollars, median, IQR)a | 410 (124–1099) |
IFI, invasive fungal infection; IQR, interquartile range; NF, neutropenic fever.
Currency: $20 Mexican peso by $1 US dollar (USD).
AMBD, amphotericin B deoxycholate.
AKI was diagnosed in 16% (39/235) of the cases during antifungal therapy, whereas LD was identified in 25% (58/235), ICU stay was required in 35% (83/238), and the mortality rate was 23% (54/229 cases) (Table 1). No difference in mortality was seen by type of proven/probable IFI (Figure S1). However, compared with possible IFIs (NF and non-NF) the mortality rate was higher for proven/probable IFIs as group (29/91, 32% vs 25/138, 18%, p=0.025) (Table S2).
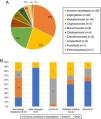
Type of IFIs, characteristics and incidence ratesThere were 98 cases of proven/probable and 140 possible IFIs, 39 in neutropenic and 101 in non-neutropenic individuals. The proven/probable IFIs were categorized into four groups: invasive candidiasis (IC), invasive aspergillosis (IA), endemic IFIs (coccidioidomycosis and histoplasmosis), and other IFIs (cryptococcosis, mucormycosis, fusariosis, non-specified IFIs). IC was the most frequent IFI overall (32/98, 33%), followed by IA (22/98, 23%), and histoplasmosis (19%) (Fig. 1A). Upon patients’ subgroups analysis, aspergillosis, was the most frequent proven/probable IFI among individuals with a hematological malignancy (18/40, 45%), and endemic IFIs (histoplasmosis and coccidioidomycosis) were more frequently seen in the HIV subpopulation (11/19, 58%) (Fig. 1B).
(A) Proven/probable infections identified during 2016 in four Mexican hospitals. Ninety-eight proven/probable IFIs were identified. Unspecified IFIs refers to diagnosis by histopathology without etiological identification. (B) Distribution of IFIs by clinical context. The frequency of the proven/probable IFIs variated depending on the comorbidity. Other IFIs includes: cryptoccocosis, mucormycosis, cladosporidosis, fusariosis, pneumocystosis, and unspecified IFIs.
The overall, IFI incidence was 4.8 cases (95% CI=0.72–8.92) per 1000 discharges and 0.7 cases (95% CI=0.03–1.16) per 1000 patients-days. IC had the highest incidence rates (1.93 cases per 1000 discharges, 95% CI=−1.01 to 2.84), followed by endemic IFIs (1.53 per 1000 discharges 95% CI=−3.36 to 6.40) and IA (1.25 per 1000 discharges, 95% CI=−0.90 to 3.45) (Table 2).
Incidence rates per 1000 discharges and per 1000 patients-days.
| Type of IFI | Per 1000 discharges (95%CI) | Per 1000 patients-days (95% CI) |
|---|---|---|
| Invasive candidiasis | 1.93 (1.01 to 2.84) | 0.36 (0.06 to 0.67) |
| Coccidioidomycosis/histoplasmosis | 1.53 (−3.36 to 6.4) | 0.24 (−0.47 to 0.94) |
| Aspergillosis and other hyalophyphomycosis | 1.25 (−0.90 to 3.45) | 0.23 (−0.14 to 0.61) |
| Mucormycosis | 0.33 (0.08 to 0.58) | 0.06 (−0.12 to 0.25) |
| All IFIs | 4.8 (0.72 to 8.92) | 0.7 (0.03 to 1.16) |
Based on these incidence rates, and according to the total number of hospital discharges (6,269,155) reported by the OCDE in Mexico during 2015, the number of new cases of IC per year would be 12,103 (95% CI=3621–20,584), for IA 7851 (95% CI=−5918 to 21,621), for endemic mycosis 9598 (95% CI=−21,034 to 40,265). Taking into account the total Mexican population (127.5 million), the incidence rate of these infections would be approximately 9.49 (95% CI=2.84–16.00), 6.15 (95% CI=−4.64 to 16.96), 7.52 (95% CI=−16.49 to 31.58) per 100,000 inhabitants for IC, IA, and endemic mycosis, respectively.
No differences in AKI or LD rates were found among the type of proven/probable IFIs, not even among survivors (Table S4). Intensive care unit admission was more frequent in patients with IC (17/32, 53%), endemic IFIs (27%, 6/22), and other IFIs (18%, 4/22). For aspergillosis, ICU stay was required only in 9% of the cases (2/22), p<0.002, no differences were found when only survivors were analyzed (Table S4).
Characteristics of the antifungal therapy usedFluconazole and echinocandins were the two most frequently indicated antifungal drug as initial therapy (Table 1). Only three out of 238 individuals did not receive the indicated antifungal therapy. Seventy-three percent of the IC cases received an echinocandin as main therapy (22/30), while for IA, voriconazole (11/22, 50%) and amphotericin B deoxycholate (6/22, 27%) were the preferred options, as for the cases with endemic and other IFIs, AmBD was the leading therapy (20/43, 46%), in these cases lipid formulation of amphotericin B was used only in 7 of 43 cases (16%).
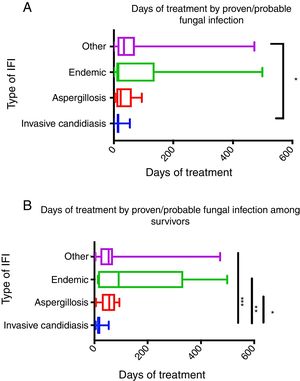
IC required the shortest duration of antifungal therapy (median 14 days, IQR 10–18) compared with the rest of the proven/probable IFIs groups (IA=median 23 days IQR 9–59; endemic IFIs=median 15 days IQR 10–91, and other IFIs=median 34 days IQR 17–68, p=0.05) (Fig. 2A). This difference was more evident (p<0.0001) when the antifungal therapy period was analyzed only among survivors. All in all, groups with longer antifungal therapy were endemic IFIs (median 91 days, IQR 14–259), followed by other IFIs (median 53 days, IQR 24–68), and IA (median 56 days, IQR 33–74) when compared with IC (median 14 days, IQR 11–22) (Fig. 2B).
(A) Time of antifungal therapy in proven/probable fungal infections in the total population. *p value=0.046. Invasive candidiasis n=32, aspergillosis n=21, endemic (histoplasmosis/coccidioidomycosis) n=21, other IFI n=21. (B) Time of antifungal therapy in proven/probable fungal infections among survivors. p value=0.002. *p=0.006 invasive candidiasis vs aspergillosis, **p=0.002 invasive candidiasis vs endemic, ***p=0.006. Candidiasis n=22, aspergillosis n=12, endemic n=11, other IFI n=14. Data presented as median and IQR. Data analyzed with Kruskal–Wallis test. Post hoc analysis was done with Dunn's test.
The development of AKI and LD was associated with higher mortality rate (39% vs 9% and 44% vs 18%, respectively, p<0.0001). These adverse events, in a multivariate binary regression analysis, were associated with death, independently of ICU stay, age, and sex (Table S5). In this model, AKI development was associated with a four-fold higher probability of dying (OR=4.29, 95% CI=1.80–10.02, p=0.001), and LD was associated with double the probability (OR=2.34, 95% CI=1.13–4.85, p=0.02).
In the overall population, the antifungal drug was modified in 80/235 (34%) cases due to different causes, such as AKI (20/80, 25%), de-escalation or adjusting to diagnosis (60/80, 75%) (Table 1).
In this study, survivors (n=175) were defined as those who received a complete antifungal therapy scheme. In this subgroup, AKI was more frequent in cases of proven/probable IFIs (11/62, 18%) than in cases of NF and non-NF possible IFIs (2/31, 6% and 3/82, 4%, p=0.01) (Table S3). This pattern was similar in all groups regarding LD during the antifungal therapy (28% vs 13% vs 12%, respectively, p=0.03) (Table S3). Among survivors who developed AKI, 31% had received AmBD and 25% LAmB as first antifungal, these proportions were higher compared with 14% and 2.6%, respectively, for the individuals without AKI (p<0.0001) (Table 3). Survivors who developed AKI received longer antifungal therapy (median 18 days IQR 14–50 vs median 10 days IQR 6–17, p=0.0008), had longer hospital stay (median 27 IQR 18–40 vs median 19 days IQR 12–31, p=0.05), and higher probability of ICU admission (63% vs 27%, p=0.008) (Table 3). The group developing LD also received longer antifungal therapy (median 15 days IQR 5–17 vs median 10 IQR 5–17, p=0.001), had longer hospital stay (median 28 IQR 20–38 vs 18 IQR 12–30, p=0.003), and ICU admission (48% vs 27%, p=0.03) (Table 4).
Characteristics in AKI and non-AKI in survivors.
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Non-AKI N=159 | AKI N=16 | p value | OR | 95% CI | p value | |
| Age, yrs (median, IQR) | 37 (21–54) | 28 (24–56) | 0.95 | 1.00 | 0.97–1.03 | 0.94 |
| Sex (female) | 76 (48) | 5 (31) | 0.29 | 0.50 | 0.12–2.03 | 0.33 |
| Comorbidities | ||||||
| Hematological condition | 48 (30) | 5 (31) | 0.8 | |||
| Solid neoplasia | 33 (21) | 1 (6) | ||||
| Diabetes mellitus | 15 (9) | 2 (12) | ||||
| HIV infection | 8 (5) | 1 (6) | ||||
| Renal transplant | 10 (6) | 1 (6) | ||||
| Other | 45 (29) | 6 (37) | ||||
| First antifungal used | ||||||
| Fluconazole | 68 (43) | 0 | <0.0001 | 24.94b | 4.2–137 | <0.0001 |
| Caspofungin | 29 (18) | 5 (31) | ||||
| AMBD | 22 (14) | 6 (31) | ||||
| Anidulafungin | 20 (13) | 0 | ||||
| Lipid formulation of AMB | 4 (2.6) | 4 (25) | ||||
| Need of a second antifungal | 48 (30) | 10 (62) | 0.01 | |||
| Time of antifungal treatment (days, median, IQR) | 10 (6–17) | 18 (14–50) | 0.008 | 0.99 | 0.99–1.00 | 0.76 |
| Length of hospital stay (days, median, IQR) | 19 (12–31) | 27 (18–40) | 0.05 | 0.99 | 0.97–1.02 | 0.365 |
| ICU stay | 43 (27) | 10 (63) | 0.008 | 17.98 | 3.2–100 | 0.001 |
| Length of ICU stay (days, median, IQR) | 14 (7–20) | 5 (2–11) | 0.02 | |||
| Total cost of antifungal drug (US dollars, median, IQR)a | 246 (9–874) | 1048 (729–6543) | <0.0001 | |||
| Total cost associated with hospital stay (US dollars, median, IQR)a | 325 (109–1003) | 680 (335–1063) | 0.04 | |||
Characteristics of non-liver damage (LD) vs LD survivors.
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| No-LD N=142 | LD N=31 | p value | OR | 95% CI | p value | |
| Age, yr (median, IQR) | 38 (21–53) | 31(26–58) | 0.68 | 1.00 | 0.97–1.03 | 0.53 |
| Sex (female) | 66 (46) | 14 (45) | 1 | 1.27 | 0.54–3.0 | 0.57 |
| Comorbidities | ||||||
| Hematological condition | 39 (27) | 14 (45) | 0.46 | |||
| Solid neoplasia | 28 (20) | 6 (19) | ||||
| Diabetes mellitus | 13 (9) | 2 (6.5) | ||||
| HIV infection | 8 (6) | 1 (3) | ||||
| Renal transplant | 10 (7) | 1 (3) | ||||
| Other | 44 (32) | 6 (19) | ||||
| First antifungal used | ||||||
| Fluconazole | 65 (46) | 3 (10) | 0.008 | 2.12b | 0.74–6.03 | 0.16 |
| Caspofungin | 26 (18) | 7 (23) | ||||
| AMBD | 19 (13) | 8 (26) | ||||
| Anidulafungin | 14 (10) | 6 (19) | ||||
| Lipid formulation of AMB | 6 (4) | 2 (7) | ||||
| Voriconazol | 7 (5) | 5 (16) | ||||
| Time of antifungal treatment (days, median, IQR) | 10 (5–17) | 15(10–46) | 0.001 | 1.00 | 0.99–1.01 | 0.12 |
| Length of hospital stay (days, median, IQR) | 18 (12–30) | 28 (20–38) | 0.003 | 1.00 | 0.99–1.02 | 0.18 |
| ICU stay | 38 (27) | 15 (48) | 0.03 | 2.92 | 1.18–7.17 | 0.02 |
| Length of ICU stay (days, median, IQR) | 13 (5–20) | 11 (7–19) | 0.98 | |||
| Total cost of antifungal drug (US dollars, median, IQR)a | 194 (8–818) | 835 (403–1407) | 0.001 | |||
| Total cost associated with hospital stay (US dollars, median, IQR)a | 337 (111–957) | 327 (123–1499) | 0.25 | |||
The cost per unit of IV fluconazole and oral formulation varied between 0.4 and 1.2 USD, which represents the antifungal with the lowest cost per unit. The most expensive antifungals, per unit, were posaconazole ($558–766) and LAmB ($154–230) (Table S1).
The cost per day of antifungal per person was $11 USD (IQR 1–104). However, the cost varied depending on the type of antifungal drug used. The median costs per treated person were $7 (IQR 4–16) for fluconazole, $654 (218–1817) for voriconazole, $731 (IQR 522–1044) for caspofungin, $612 (IQR 349–1136) for anidulafungin, $96 (IQR 36–375) for AmBD, and $6840 (IQR 3040–9120) for LAmB (p<0.0001).
When the cost of antifungal therapy was analyzed by type of proven/probable IFI, among survivors, the group of other IFIs had the highest cost per treated person ($1843, IQR 567–46,543) followed by IA ($1382, IQR 645–2924), and IC (1072, IQR 528–1461), whereas endemic IFIs showed the lowest cost per treated person ($266, IQR 163–546), p=0.015 (Table S4).
Patients with AKI needed a second antifungal more frequently (30%) than those without AKI (54%) [p=0.008], and this difference was more evident in individuals who survived (30% vs 62%, p=0.01) (Table 3). The presence of AKI increased 4.3-fold the cost of antifungal therapy (median $246 IQR 9874) when compared with individuals without AKI (median $1048 IQR 729–6543) [p<0.0001] (Table 2). This increase in cost was mainly due to the cases with possible IFIs, between 7- (for cases of NF possible IFIs) and 18-fold ((for cases of non-NF possible IFIs) when all cases were analyzed (Table 5). However, the costs increased between 12- and 31-fold when the analysis was restricted to survivors, for non-NF and NF possible IFIs, respectively (Table 5).
Increasing cost associated with AKI development during antifungal therapy.
| All population | Cost of antifungal treatmenta | p valueb | ||
|---|---|---|---|---|
| Condition | Proven/probable IFI | NF possible IFI | Non-NF possible IFI | |
| Non-AKI (n=180) | ||||
| Median | $907 | $75 | $28 | <0.0001 |
| IQR | (178–1843) | (7–192) | (6–642) | |
| AKI (n=38) | ||||
| Median | $343 | $512 | $524 | NS |
| IQR | (7–1875) | (111–858) | (112–2384) | |
Also, LD had a 4-fold increase in the cost of antifungal therapy (Table 3). Similarly, to AKI, the costs increased for non-NF and NF possible IFIs cases between 30- to 58-fold, and between 35- and more than 200-fold increase among survivors with non-NF and NF possible IFIs cases, respectively.
Mexican health system estimated expenditure in antifungal therapy in a one-year spanInformation about the costs of the antifungal therapy and length of hospital stay provided by each hospital and case-specific allowed to determine the mean cost of a day of antifungal therapy and hospital-stay (Table 6). These values could vary, depending on the type of antifungal drug (more expensive for liposomal amphotericin B and less expensive for generic formulations of fluconazole), required doses, ICU stay, and if the patient died or survived. In order to have an approximated mean cost of the antifungal therapy, all different drug costs for all patients were taken into account, without distinction with regard to the final outcome (Table 6).
Antifungal and hospital stay costs of IFIs in Mexico.
| Costs per daya | Value | ||
|---|---|---|---|
| Daily cost of antifungal therapy Mean value ($), 95% CI | 93 (61–124) | ||
| Cost per day in hospital Mean value ($), 95% CI | 25 (19–31) | ||
| Daily ICU cost Mean value ($), 95% CI | 90 (67–112) | ||
| Treatment duration, days (mean, 95% CI) | 25 (17–33) | ||
| Type of IFI indicating antifungal therapy | Proven/probable IFI | NF-possible IFI | Non-NF possible IFI |
| Estimated number of people receiving antifungal therapy in one yearb (number, 95% CI) | 30,091 (4396–55,785) | 12,109 (1769–22,450) | 31,191 (4556–55,785) |
| Treatment duration, days (mean, 95% CI) | 48 (30–66) | 11 (8–14) | 9 (7–10) |
| Rate of AKI | 27% | 10% | 9% |
| Rate of LD | 34% | 26% | 15% |
| Increasing antifungal cost due to AKI | –c | 12 times | 31 times |
| Increasing hospital stay cost due to AKI | 2 times | 2 times | 1.8 times |
| Proportion of increasing antifungal cost due to LD | c | 35 times | 200 times |
| Proportion of increasing hospital stay cost due to LD | –c | 2 times | 2.7 times |
| Rate of ICU stay | 29% | 15% | 47% |
| Increasing hospital stay cost due to ICU stay | 3 times | 2.5 times | 7.5 times |
Assumed cost and associated increasing were taken from the results obtained in the clinical and cost analysis presented in previous sections in this manuscript.
Taking into account incidence rate of IFIs (4.8 per 1000 discharges, 95% CI 0.7–8.9) and number of discharges during 2015 in Mexico. Number of discharges during 2015=6,269,155 (source: stats.oecd.org). Proven/probable IFIs was considered to be 41% of people receiving antifungal, NF-possible IFI 16% and non-NF possible IFI 42% of people receiving antifungal considering the results of this paper in Table 1.
The total number of hospital discharges in Mexico was obtained from the OECD webpage.12 The last information available corresponded to 2015 for the hospital discharges by diagnostic categories. A total of 6,260,155 hospital discharges were reported by OECD during that year. Taking into account, the proven/probable IFI incidence rate estimated in this study, 4.8 per 1000 discharges (95% CI 0.7–8.9), we estimate that in one year the total number of proven/probable IFIs treated with an antifungal drug to be approximately 30,091 patients (95% CI 4396–55,785) (Table 6). However, based on our data (showed above), these numbers correspond only to 41% of the cases receiving an antifungal drug (Table 1). Hence, the total number of individuals who received an antifungal drug due to any type of IFI (proven/probable/possible) in one year would be 66,510 individuals (95% CI, 3930–130,602). This estimated burden represents an approximation to the total use of antifungal therapy in Mexico.
Taking into account the data expressed in this study, the estimated mean duration of one antifungal therapy for any type of IFI was 25 days (95% CI 17–33). The mean cost of one treatment, therefore was $1100 USD (95% CI 600–4488). However, the duration is usually longer in proven/probable IFIs (Table 6), especially for IA and endemic IFIs (>400 days in some cases) (Fig. 2). Hence, this estimate shown above could be even higher.
The total expenditure in antifungal therapy for all type of IFI, without adjusting for AKI, LD and ICU stay, would be approximately $154,636,516 USD (95% CI $4,075,571–$534,423,801 USD). After adjustment only for the proportion of expected individuals to develop AKI and the increasing in antifungal cost in such cases, the expenditure in antifungal therapy for all type of IFI would ascend to $233,435,536 USD (95% CI $6,224,993 to $773,810,330).
DiscussionHerein we report the characteristics of IFIs managed in four hospitals in Mexico and provide an estimate of the burden of IFIs in Mexico. The overall incidence rate of IFIs and more specifically the individual rates of aspergillosis, histoplasmosis, coccidioidomycosis and candidemia are shown here and, with the exception of invasive candidiasis, no similar study has been published in recent years.13,14
Prior studies on coccidioidomycosis and histoplasmosis from Mexico have focused on the prevalence of these two infections by using coccidioidin skin testing15 and not focused in the incidence of active infections.
In Mexico, notification of these IFIs to the national health ministry was discontinued in 1995.16 The last incidence rate reported for coccidioidomycosis and histoplasmosis in Mexico was 1.3 and 0.3 cases per 100,000 inhabitants, respectively.16 During 1995, in the US, the incidence of coccidioidomycosis was 1.9 cases per 100,000, which increased, since 1998, from 5.3 per 100,000 to 42.6 in 2011 and to 8.82 in 2015.17,18 Meanwhile, histoplasmosis incidence rate was maintained steady between <1 and 1.7 cases per 100,000 between 2001 and 2012.19,20
In the current study, we estimated an incidence rate for endemic IFI of 7.5 per 100,000 inhabitants although it is possible that in Mexico, as for coccidioidomycosis in the US, the number of cases of these infections have increased since 1995.
Diagnostic improvement in IA has been in part due to the availability of galactomannan antigen detection in specialized reference centers, not only in Mexico, but in other Latin American countries.21 This is the first study reporting the incidence of aspergillosis in a Mexican population where rates are similar to previously published incidence rates in the US during 2009–2013, estimated to be 2 cases per 1000 discharges among individuals at risk.22
In this study, that at least 30,000 new cases per year of proven/probable IFIs were estimated to have occurred in Mexico. This estimation would be higher than the number of new cases officially reported by the Mexican health department during 2016 for Chikungunya, hemorrhagic Dengue, and Zika virus infections (n=13,030 cases), hepatitis A, B and C virus (n=10,456 cases), HIV asymptomatic infection (n=7333 cases), and tuberculosis (n=20,811 cases).16 We believe that with the estimated cases expressed here, IFIs should become a notifiable group of diseases and be treated as a public health problem.
The four hospitals included in this study have several differences, such as number of beds, patient diversity, geographic location, and policies about the reimbursement of expenses, mainly for antifungal drugs. The data presented in the study is heterogeneous, however, this heterogeneity is a rule more than an exception in Mexican hospitals. Nevertheless, we acknowledge limitations in the estimations showed in the current study, as these four hospitals only represent 0.3% out of the total hospitals in the public health care system and 0.5% of hospitals in the public health care system for those without institutional insurance, in Mexico.23 These limitations could be surpassed if the notification of these infections becomes mandatory, and the access to better diagnostic tools is improved.
The diagnosis of possible IFIs, receiving empirical therapy, corresponds to more than half of the cases. This proportion can be the result of several phenomena. First, most of the proven/probable IFIs were diagnosed via culture techniques which are a low sensitivity diagnostic tool for IFIs. A classic example is IC, as blood culture identifies only two to seven out of 10 cases.24 The capacity to diagnose this infection increases to eight to nine out of 10 cases when non-culture techniques are added to the diagnostic armamentarium, such as PCR and β-d-glucan assay.24 In the case of histoplasmosis, the inclusion of training in diagnosis by direct examination, pathology and PCR showed a three-fold increase of reported cases in the French Guyenne as well as a decrease in morality due to this infection from 40% to 10%.25 In Mexico, as in other Latin American countries, culture-based diagnosis is not always available at second care level hospitals, expertise in histological examination of fungi is limited to high specialized referral centers, and non-culture techniques, other than galactomannan, are not available even at referral centers. Second, as the lack of access to better diagnostic tools decreases the possibility of accurate identification of IFIs, this also, may increase overdiagnosis and hence overuse of empiric antifungal drugs, causing unwanted adverse events such as AKI or LD, and higher costs for medical institutions and patients, as shown in this study.
Amphotericin B formulations, itraconazole, voriconazole, flucytosine, and fluconazole are in the Essential Medicine List issued by the WHO.26 Except for flucytosine, these drugs are all listed as national essential drugs in Mexico.26 In the participating hospitals in this study mostly of these drugs were available, except for LAmB, at different costs.
According to the Mexican universal list of health services (CAUSES), AmBD is considered essential in the management of fungal meningitis and pneumonia.27 During 2016 and 2017, amphotericin B deoxycholate became unavailable across the country, which led to a switch in the pattern of antifungal management. One of the changes brought by the lack of AmBD, during that period, was the inclusion of LAmB at a catastrophic cost. However, access to this drug is still problematic, not all patients are covered and, if it is needed, the cost is absorbed by the patient. As showed here and in previous studies,28 the cost of LAmB per unit is at least 10-fold more expensive than AmBD, and usual dose per day is three-fold higher, >$300 USD per day, which is 75-fold higher than the daily minimum wage in Mexico.29 Besides the economic cost of antifungals, access is also a concern, owing to the limited number of manufacturers and/or distributors of these drugs.30 In our country, currently, only two companies distribute generic AmBD and two distribute LAmB. At least five are licensed to distribute generic fluconazole, and one patent fluconazole.31 Unavailability of either amphotericin B or flucytosine impacts the prognosis of infections such as cryptococcus, histoplasma and mucormycosis meningitis, in addition to some resistant aspergillosis.30,32,33
In this report, the estimated expenditure only with antifungal drugs in one year would represent 29% of the required annual budget to manage complications due to obesity.34 The cost of antifungal therapy estimated in this study did not include costs associated with hospitalization, blood, and imaging tests, which are necessary to have a better panorama of the expenses due to IFIs. It is known that IFIs are an independent factor for higher associated health care costs.35 Antifungal drugs increase at least 25% these costs and hospitalization costs can be 40% higher when an IFI is diagnosed, mainly for invasive candidiasis, aspergillosis and mucormycosis.36 In-hospital LOS is longer for cases with IFIs. In our study, LOS was almost two-fold longer for aspergillosis and endemic mycosis when compared with reports from other countries.37
This is the first study conducted in Mexico and Latin America analyzing the impact of AKI development during treatment of IFIs on the cost of antifungal therapy, LOS, and mortality rates. As previous reports have shown, in our study AKI was associated with higher mortality rate during IFIs therapy, usually associated with the use of amphotericin formulations.38 Previous studies have identified AKI as independent factor associated with death in hospitalized patients, with a probability of death 3 to 26 times higher, similar to our findings.39 As previously shown, AKI inflates the cost of the IFI therapy, between 1 and 7 times.38,39 In our data, such an increase was not restricted to AKI as LD also impacted the overall cost.
In this report, other contributing factors to AKI or LD were not assessed, which is a limitation inherent to the study design. Most of the individuals suffering from an IFI require multiple medications to treat comorbidities such as chemotherapy, antiretrovirals, or have underlining AKI such as diabetic patients, and could also be critically ill due to IFI or other conditions. We could not ascertain if AKI was secondary to the use of antifungal therapy since we did not control for other confounders, but we could establish the high burden and impact of AKI in patients with IFI, which justifies increasing focus on preventive measures.
ConclusionAwareness of the burden of IFIs in Mexico and the estimation of the treatment cost per person, allowed us to have an approximate expenditure in antifungal drugs by the health care system. This estimation allows to assess cost-avoidance strategies, such as antifungal prophylaxis, antifungal stewardship, programs improving access to essential drugs and make cost-conscious decisions such as access to more diagnostic tools, to increase diagnostic-driven therapy and decrease unwanted adverse events and their associated cost.
ContributionsDECL contributed to the conception and design of the study, also to the analysis and interpretation of data, and drafting the manuscript. DPM, AMO, NRM, HVL contributed with acquisition of data, and drafting the manuscript. AMO and ACO contributed with critical revision of the manuscript, interpretation of data and drafting the manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.