Vaccines are well-established public health interventions with major impact on the prevalence of infectious diseases, but outbreaks are occurring frequently due to primary and secondary failures, despite high coverage. Surveillance of efficacy and duration of induced immunity is a difficult task as it requires invasive blood sampling in children and teenagers. Saliva can be an acceptable alternative source of IgG to assess vaccine efficacy and toxoplasmosis incidence. We investigated IgG response for measles, mumps, rubella, and T. gondii in saliva samples of vaccinated young people.
MethodsSaliva was collected from 249 public schools students from São Paulo, Brazil, aged 7 to 13 years old, during an interactive exhibition on hygiene. We used S. aureus protein A solid phase capture assay for IgG reactive to biotinylated purified proteins. Paired saliva and serum (47) were tested from young adults with serum evidence of T. gondii infection and from negative children less than 12 month old for standardization. Reproducibility was greater than 98% and sensitivity and specificity of the saliva assays were greater than 95%, as well as the concordance of paired saliva and serum samples.
ResultsSaliva from high school students showed a prevalence of 8.5% (95% CI: 5.0–11.9%) for anti T. gondii IgG; 96.8% (94.6–99%) of anti-measles IgG; 59.1% (53–65%) of anti-rubella IgG, and 57.5% (51.3–63.6%) of anti-mumps IgG.
DiscussionThe prevalence of antibodies against mumps and rubella after 6–8 years of vaccination was lower than against measles among students. The findings of this study demonstrate the feasibility of saliva sampling for follow-up of vaccine immune status in teenagers. This useful approach allows for IgG detection for vaccine control or epidemiological studies.
The use of safe and effective vaccines are well-established and cost-effective public health interventions over the last three decades and with a major impact on the prevalence of infectious diseases.1 Usually restricted to children, benefits of immunization are increasingly extended to adolescents and adults, providing protection against life-threatening diseases such as influenza, meningitis, and cancers that occur in adulthood.2 Despite this progress, vaccine-preventable diseases continue to be a major cause of morbidity and mortality. Cultural and fake news on association of vaccines and diseases have induced higher rate of non-vaccinated children due to parental concern.3 Some authors have demonstrated that a lack of response to the vaccine, primary vaccine failure (PVF), may occur in about 2–5% for measles, 3–7% for mumps, and 2–5% for rubella.4,5 Among the main causes of PVF are the presence of maternal antibodies and improper vaccine storage and management (handling, administration, cold chain). Secondary vaccine failure (FVS), which is the drop in immunity over time expressed as low or even undetectable antibody levels, thus failing to confer protection.6–8 For these reasons, even with an effective children immunization program, as reported in our region,9 a growing number susceptible individuals can be found after a few years as a result. Therefore, vaccination coverage may not be equivalent to population immunity.10,11 The triple viral vaccine (MMR) is prepared from live attenuated measles virus (Schwarz, Moraten and Edmonston Zagreb strains), mumps (Jeryl Lynn, L-3 Zagreb and Urabe AM9 strains) and rubella (Wistar strain RA27/3). A study conducted in Finland showed that antibody levels induced by the MMR vaccine decrease progressively after the second dose. The protection induced by this vaccine seems to persist at least until the beginning of adulthood; however, the situation requires constant vigilance.12 Antibodies against measles, mumps and rubella decrease on average 3% per year and show a high degree of individual variation. The antibody decay rate varies substantially between individuals and between the three groups of antigens present in the MMR vaccine. However, higher rate of decline along with high variation is observed with mumps.13 Prevalence studies of measles, mumps, and rubella antibodies are not only aimed at measuring the proportion of the immune population, but rather provide data to support new immunization strategies to be introduced to control possible vaccine failures and predict the occurrence of outbreaks, as commonly seen in our country.14
Because of technological and laboratory advances in oral fluid studies, saliva tests besides detecting diseases related to oral health may be used as markers of the general health of the individual.15,16
With these advancements, early disease monitoring and detection17,18 have played a key role in the success of therapy and may reduce the severity and complications of diseases. The main tool for the surveillance and monitoring of vaccinated children is related to the increase of expedite and non-invasive diagnostic tests and help preventing possible outbreaks.19,20 The advent of oral fluid testing has transformed measles surveillance in England and Wales. With a sensitivity of 92% and a specificity of at least 98% in the IgM test with oral fluid, it is suitable for surveillance.21 It is known that the measles vaccination failure rate ranges from two to 10%, and it is an important issue in the epidemiology of the disease, since the transmission could only occur due to failures in childhood vaccine coverage.22 Recent studies have shown that children are the primary targets for measles and, in this non-invasive technical context for diagnosis, saliva can be a very useful tool in early detection of measles. The results confirm that both serological and molecular assays of oral fluid are suitable for routine use.23 The variable amount of IgG in saliva is the main problem for the detection of specific IgG which may result in false negative results, but we have developed an effective assay for the detection of anti-T. gondii IgG, using the capture of a fixed amount of IgG in the solid phase revealed by biotinylated antigen.24 In the present study, we investigated the presence of specific IgG against measles, mumps and rubella or toxoplasmosis in saliva of teenagers from public schools. Undergraduate students from our University provided paired samples of blood and saliva to standardize the method.
Material and methodsStudied populationA total of 249 samples of saliva from elementary school students and 47 sera and 47 saliva (paired) from undergraduate students were collected. In addition, 50 sera from our biorepository known to be positive for the diseases studied and 40 negative samples from 3 to 5 pooled discarded sera from infants between eight and twelve months of age from the Children's Institute of the Hospital das Clínicas of the São Paulo Medical School (IC-HCFMUSP) were also used.
All volunteers, high school and undergraduate students, were adequately informed of all procedures to be developed, with signed individual or parental consent form, according to Resolution No. 196/96, which regulates research involving human beings. All high school students were also informed and consented in individual participation, aside to approved parental consent. This study was submitted to the Research Ethics Committee and approved under process nº 23109613.1.0000.0065.
Saliva collectionSalivette commercial tubes without astringent were used for saliva collection containing an absorbent cotton roller and a tube suitable for saliva centrifugation after collection. The students were instructed to mouthwash with 25–40ml of oral rinse (Listerine®), followed by mouthwashes with drinking water, insertion of the cotton roller of the annotated device in mouth without touching, chewing until appears completely moistened and reinserted in the tube, which was stored in ice bath until centrifugation. The tube was centrifuged at 2000g for 10min at 4°C and the cleared saliva transferred to a 2ml conic tube, with addition of 1 volume of −20° C absolute ethanol. The solution was vortexed and maintained over night at −12°C. The precipitated IgG was recovered by centrifugation at 10,000g for three minutes at -−2°C, drained and dissolved in 1/5 of the original saliva volume with saline borate buffer.
Immunoenzymatic assaysWe have developed IgG immunoenzymatic assays using capture of IgG by solid phase protein A and subsequent reaction with in-house biotinylated recombinant viral antigens,24 revealed with avidin-peroxidase (supplementary data). 96- or 384-well polystyrene plates wells were absorbed with a fixed amount of protein A allowing for a fixed amount of IgG present in saliva or serum to bind evenly through its Fc fragment, independent of its concentration in saliva. The subsequent reaction with viral or T. gondii antigens conjugated to biotin allows the detection of the proportion of specific IgG in the sample, with avidin-peroxidase and development by TMB. Adequate controls were used in each plate and IgG levels were expressed as Artificial Units, as a ratio of observed O.D. in sample and cut-off O.D.
Statistical analysisAll data were analyzed using a Graph Pad Prism 5.0 software. ROC curves and serological indexes were calculated using negative and positive standards confirmed by other tests. Between groups differences were considered significant when p<0.05. All frequency data were presented with its 95% confidence interval. Reproducibility assays and sera and saliva comparisons were performed in different days with different microplates with the same samples and compared by Pearson correlation.
ResultsHigh school samples vaccination status and demographyThe prevalence of antibodies against T. gondii and the antigens of the triple viral vaccine in students from the city of São Paulo was assessed after a decade from two doses of live MMR vaccine. Parental consent was obtained from 263 students, but 14 students refuse to participate in the study, despite parental consent. All students participated in the hygiene exposition, regardless of parental consent or their own consent to participate in the study. Vaccination coverage of the study sample was excellent, according to the vaccination card recovered from 82.3% of the students, a very reliable instrument. However, a significant fraction had not completed vaccination, although all had taken at least one dose of the vaccine recorded in the card. This is the usual vaccination coverage in São Paulo, a state with over 95% MMR coverage. There were 160 girls and 89 boys, a ratio similar to the student population with girls more adherent to high school than boys, a fact caused by their sociocultural background and family economic status. Regarding toxoplasmosis risk factors, 38% refered ingestion of raw or crude meat, with only a few vegetarians (3.2%), mostly ingesting only well-cooked meat (57.8%).
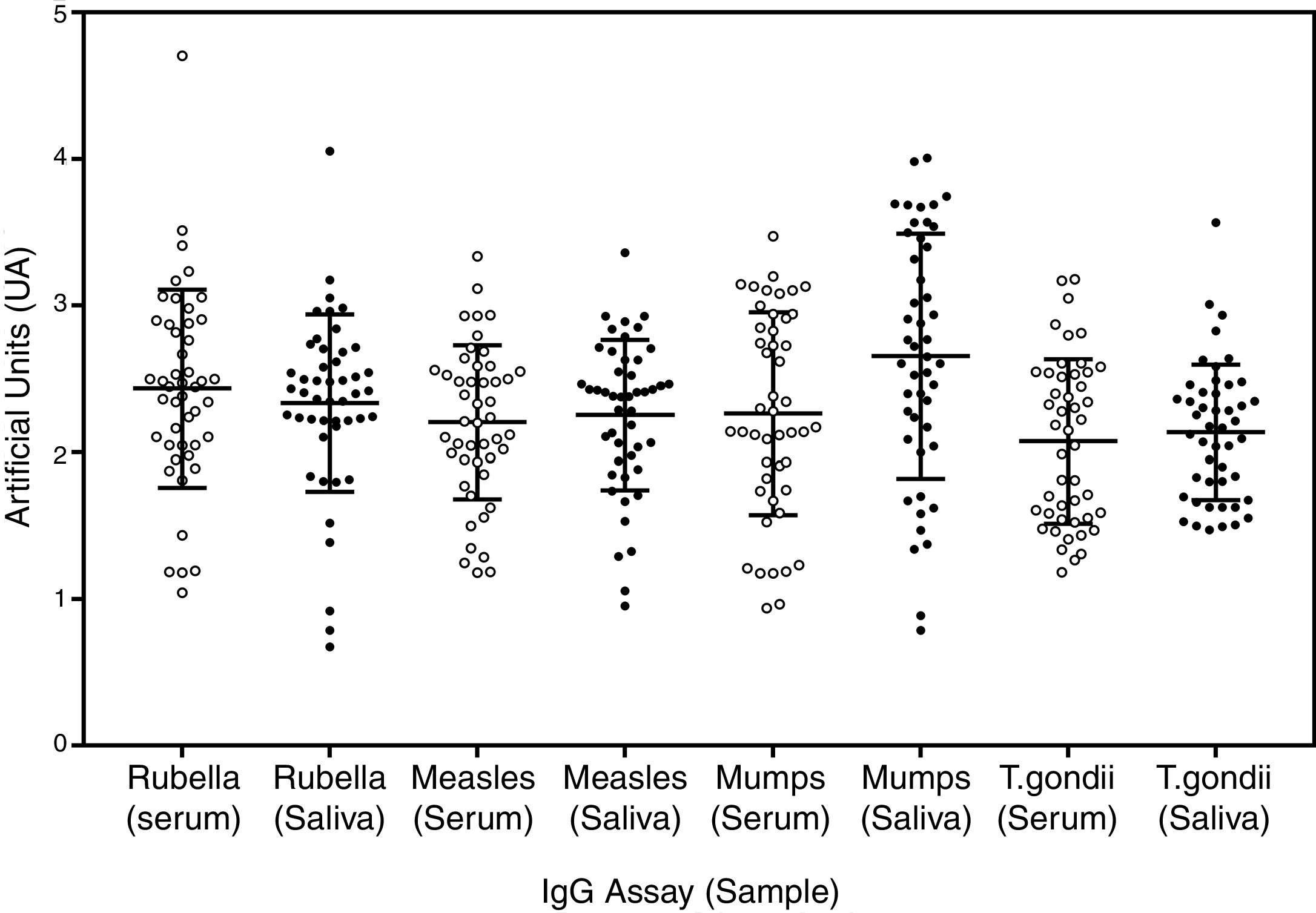
Standardization of saliva testsA total of 49 paired serum and saliva samples and adequate undergraduate adult students controls in our assay, using all the biotin labelled antigens (supplementary Fig. S1), were used to verify the reliability of saliva assays. As it can be seen in Fig. 1, there was no significant quantitative difference between serum and saliva IgG with the capture protein A assay. The qualitative results obtained for serum and saliva samples presented the similar proportions, but a small number of negative vaccinated samples was detected with some antigens, like mumps (2/47) and rubella (3/47), but these samples were also of low reactivity with serum, with border-line results. The most important result is the absence of difference and the reliability of both IgG samples, showing that the use of capture protein A IgG assays is a feasible method for detecting specific IgG for the studied antigens in saliva samples. Data on antigen quality, reproducibility, and quantitative comparison between serum and saliva, can be seen in the section of Supplementary data. All reactions had a significant correlation between serum and saliva (Supplementary Fig. S2). There was high reactivity of measles and rubella samples, with high OD and consequent blurring beyond the accuracy of microplate reader, causing a less intense correlation, but all positive samples presented qualitative concordance regarding the presence of specific IgG, independent of the source.
Paired serum and saliva samples on plate adsorbed with protein A and with biotinylated antigens. The type of antigen is expressed at the bottom of the graph with sample type between parentheses. Open dots are serum samples and closed dots saliva samples. Artificial units (UA) represents the ratio of the reactivity of each point and 95% cutoff of negative samples in the same plate. The error bars represent the mean and standard deviation of each population studied.
High school students’ saliva samples were tested only after confirming saliva as a reliable source of specific IgG. The gold-standard for comparison in this detection was undergraduate students’ saliva, which had paired serum-saliva samples, used as positive controls. As negative controls, we use pools of four negative sera (75) of unvaccinated 8–12 month-old children. We evaluated the efficiency and reproducibility of our protein A capture assay and biotinylated antigen by comparing the qualitative reaction by two different reactions, performed with the same saliva dilution at different days (Supplementary Fig. S3), which shows excellent reproducibility with an R2>0.95 and low dispersion of the data.
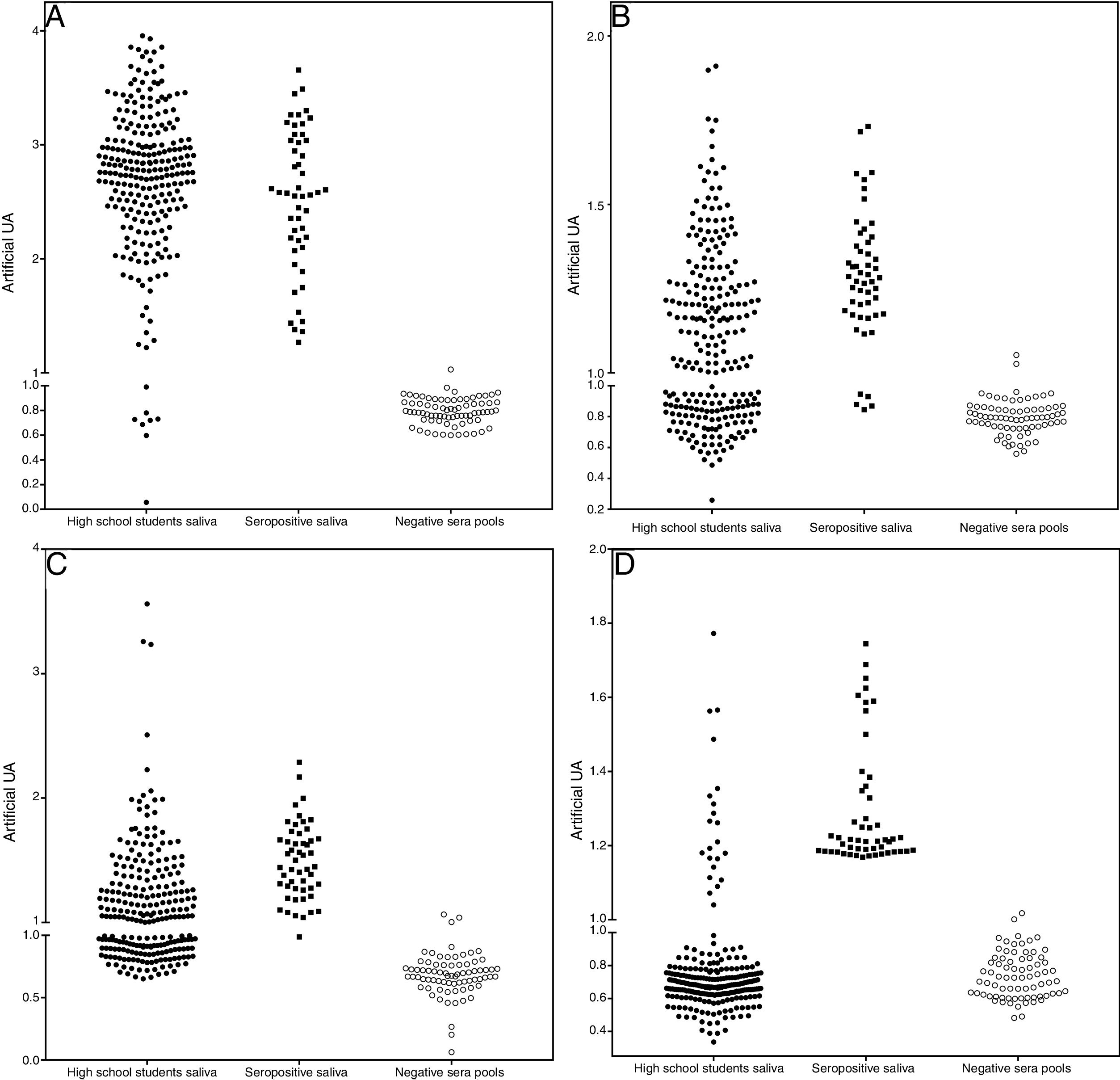
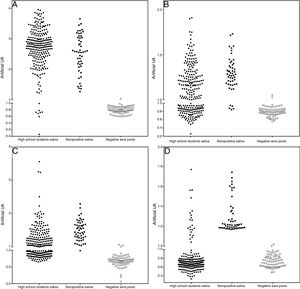
The assays for determination of IgG specificity by biotinylated antigens after solid phase capture with protein A showed a clear distinction between positive and negative sera for the four types of antigens analyzed. This allowed us to infer the prevalence of these antibodies in the studied population of preadolescent children. Quantitative analysis shown in Fig. 2 depicts excellent quantitative discrimination among students, using saliva as the source of antibodies. As for measles, the students maintained a high frequency of positivity for anti-measles IgG antibodies, which was not the case for the other viruses, where the positivity rate was lower, as was the frequency of detectable level of specific IgG (p<0.001). There was no difference in the frequency of positivity between rubella and mumps. For toxoplasmosis, there was a much lower rate of positivity among the students, and there was no reason to compare this infection with the viral antibodies resulting from vaccination, which only represents the contact of those students with that pathogen. Aside from this quantitative analysis, we performed a ROC curve analysis for defining the best cut-off (Supplementary Fig. S4). Using this cut-off, it was possible to estimate the frequency of positive samples among high school students, as shown in Table 1.
Distribution of IgG detection results by capture ELISA in saliva samples from high school students, compared to saliva from positive paired samples and negative sera. Capture IgG ELISA reactivity expressed as artificial units (U.A.). Vertical axis interrupted at 95% cut-off of negative samples. A – Measles, B – Rubella, C – Mumps, and D – T. gondii STAG.
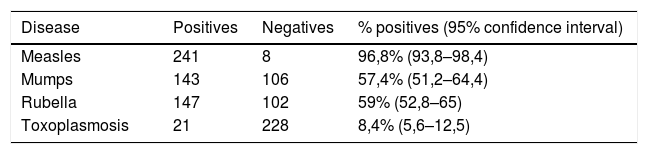
Qualitative frequency of positivity in school saliva in the protein-specific IgG capture assay and reactivity with biotinylated antigens, after ROC curve analysis.
| Disease | Positives | Negatives | % positives (95% confidence interval) |
|---|---|---|---|
| Measles | 241 | 8 | 96,8% (93,8–98,4) |
| Mumps | 143 | 106 | 57,4% (51,2–64,4) |
| Rubella | 147 | 102 | 59% (52,8–65) |
| Toxoplasmosis | 21 | 228 | 8,4% (5,6–12,5) |
In this work, we aimed to study if saliva could be used as a source of specific IgG in a vaccinated sample of high school students. Adolescent people have adequate comprehension but not legal responsibility for participation, demanding parental consent. This approval demands several contacts for information and, despite the efforts of teachers and school staff, there was low response rates in public schools, as the parents had no interest or were busy precluding adequate analysis. Adhesion to the study was compromised by the need of blood collection. As an strategy to increase participation, we promoted an interactive exposition on hygiene and infections transmission ways to foster voluntary participation. Saliva collection is much more friendly and cheaper, but previous studies had reported small fractions of false negative results due to low IgG content in saliva24 or proteolysis of antibodies.24 Towards reducing those problems, we had reported a protein A capture assay to reveal specific IgG against T. gondii in saliva,24 which minimized false negative results. This result is expected due to the biological activity of protein A, which binds to the Fc of two IgGs, presenting to the antigen four binding FABs, thus resulting in a sensitive assay with low IgG levels, as expected in saliva. The assay was also performed with cleared and ethanol precipitated saliva, bringing the IgG concentration to one-fifth of the original saliva volume.
Thus, a 10ng binding of protein A results in the binding of 90ng of IgG to the microplate surface and increased by almost 10 times the absorption of IgG in the solid support. Several other chemical methods have been used to adhere IgG directly to support solid such as those used in plasmon resonance and other detection methods,25 but the capture proposed in this study provides comparatively more FABs per area for specific detection, which seems ideal for detection of specific IgG. Non-capture of antigens by monoclonal antibodies demands more steps and reagents.24
Our data were equally efficient and reproducible for all antigens. Initially, the assays were tested with serum that had excess IgG compared to the amount of IgG in saliva.23 As expected, salivary IgG reactivity was very similar to serum IgG reactivity, as evidenced in all of our assays. This finding was certainly related directly to the use of protein A capture in the solid support, which standardized the IgG supply for reaction with the biotinylated antigen. In fact, we are measuring the proportion of IgG reactive to the antigen, not the amount of specific IgG in serum, similarly to non-quantitative ELISA.
The IgG capture approach, in addition to ensuring a uniform amount of IgG, also decreased the risk of competition with other immunoglobulins more frequently present in saliva, such as IgA, which could lead to false negative results by blocking the IgG reaction in the presence of large specific IgA titers. Antibody competition was mentioned by authors who worked with dengue serology on several biological materials.26 The low prevalence of toxoplasmosis found in our study was expected as previous studies suggested that the incidence of the disease in our area is decaying among the youth.24
For mumps antigen, the failure of the original biotinylation was due to this recombinant antigen being less soluble and urea was used in the original preparation, which residual contamination prevented correct labelling. The option found was biotinylating in diluted solutions after molecular exclusion chromatography to remove all low molecular weight contaminants in which we were successful. However, it prevented Western blot execution due to the extreme dilution of the biotinylated antigen. In this way, all of our antigens showed excellent marking and allowed continuity of the assay development.
Our data showed several important aspects of the triple viral vaccination. Most importantly, measles vaccine coverage in our group is relatively maintained, while protection for rubella and mumps was less durable with a significant fraction of lost protection, which might explain the recent outbreaks of these viruses in our country. Therefore, there is a need for additional vaccination doses during adolescence, an important sanitary measure for the prevention of congenital rubella.27
Our data corroborate previous reports of preferential loss of immunity against mumps and rubella compared to measles. There are different mumps virus used in vaccines with varying degrees of efficacy.28 The worldwide triple viral vaccine has variable rates of success and there have been few recent quality control investigations. In a recent review, Plotkin et al. discuss several aspects of the concomitant immunogenicity of viral infections preventable by MMR. There is a correlation between antibodies and protection for measles and rubella, but few studies have been conducted with mumps, and even in this review, there are no reports of efficacy evaluation of this vaccine.29
Our study shows a possible shorter duration of protection against the two less dominant viruses (rubella and mumps) in our population and the explanation for that is complex. In a natural infection, measles virus induces host immunosuppression, as assessed by lung histology in children.29,30 In contrast, measles vaccine appears to affect the immunity of vaccinated persons by increasing resistance to infections. Interestingly, the disappearance of immunity has been reported for only two vaccine secondary viruses,31 as found in our study.
There are no reports of constant quality control of commercial preparations of the vaccine and there may be more environmental resistance of the measles virus. Thus, it was not unexpected that immunity to secondary vaccine viruses would decline, as short-term reports had already demonstrated this problem.32 The need for prevention of congenital rubella syndrome has been of major concern and therefore revaccination in with the triple viral vaccine is recommended in adolescence,30 as has been conducted in our state.
Resurgent outbreaks of vaccine preventable diseases previously controlled or eliminated have been observed in many contexts. Vaccination campaigns can prevent or even control outbreaks but must be worked with considerable certainty, and real-time surveillance can provide valuable information about the population at risk and who would be the primary targets for vaccination to block potential outbreaks. However, we are faced with preventive diagnostic limitations, since ideally the immunological status should be confirmed for each individual, before an unnecessary revaccination,32,33 in areas with very good immunization coverage.9
Our approach was efficient in all aspects, from the interactive exhibition for better compliance with saliva collection to the development of reliable capture ELISA to be used with saliva for determination of vaccine immune status or toxoplasmosis immunity among public high school students. In conclusion, we can properly plan adequate public health measures both individually or as group, such as individual revaccination in case of loss of vaccine immunity or estimating real vaccine coverage in larger groups.
Conflicts of interestThe authors declare no conflicts of interest.
This experimental work was supported by Fundação a Pesquisa no Estado de São Paulo/SP/Brazil (FAPESP Process 13/04676-9). This work was the experimental part of Ph.D. thesis in Tropical Medicine/USP of Barbara Carvalho Fialho Sampaio. B.C.F. Sampaio and J.P. Rodrigues were fellows of Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) for the Ph.D. Program in Tropical Medicine/USP. L.R. Meireles and J.P. Rodrigues leaded the interactive exposition with participation of Gabriela Marques and Yugo Gushiken. This exposition was financed by the Pró-Reitoria de Cultura e Extensão Universitária, USP, Brazil. LIMHCFMUSP partially supported infrastructure of this research.