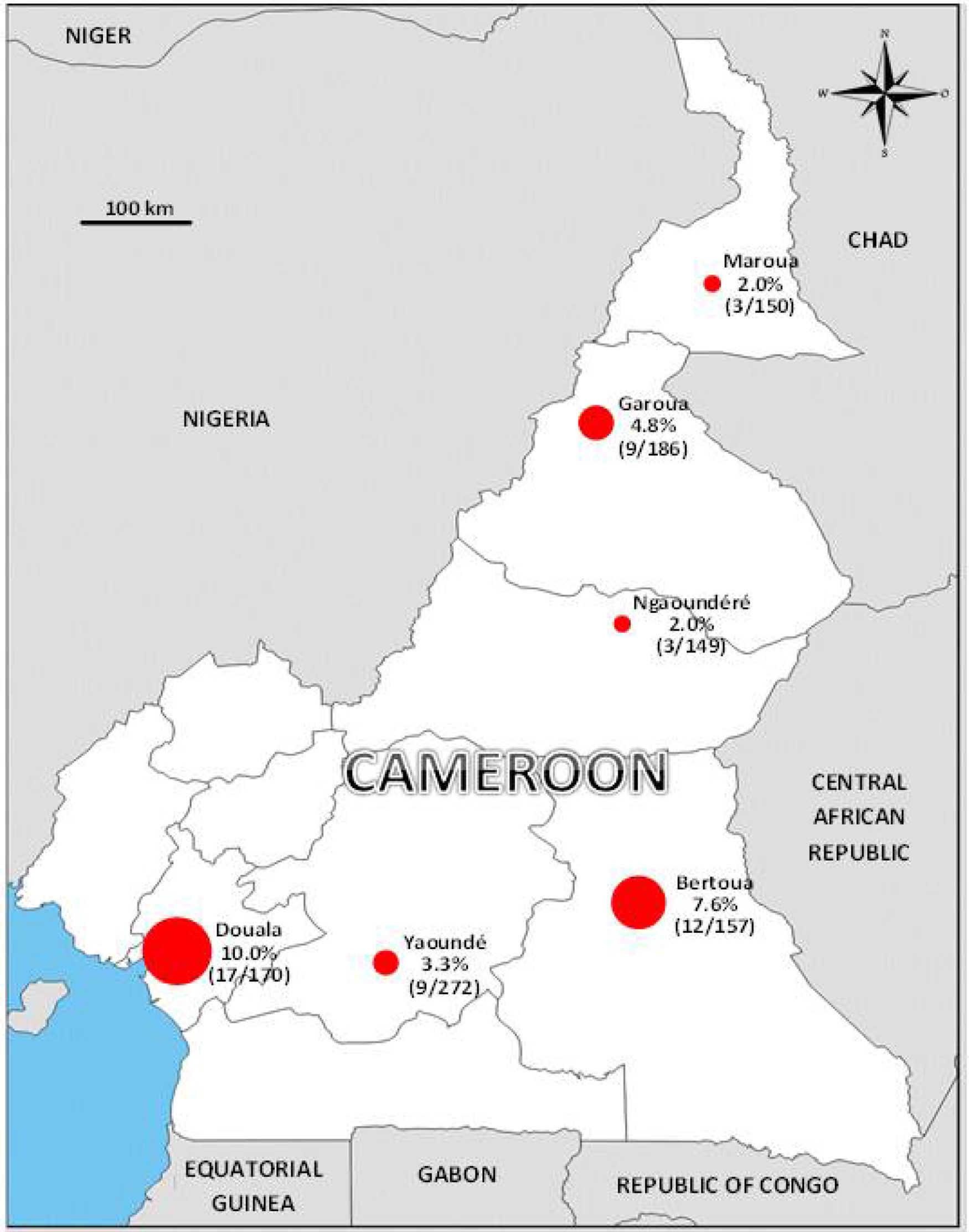
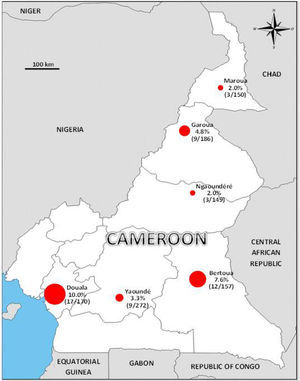
A Zika virus seroepidemiology study was performed in 1084 blood donors collected from August to October 2015 in six sites of Cameroon representing a large panel of eco-environments. Samples were tested using an anti-NS1 IgG ELISA detection kit and positives were further confirmed by seroneutralization. The observed global seroprevalence was low (around 5%, peaking at 10% and 7.7% in Douala and Bertoua, respectively) with risk factors associated with seropositivity pointing to the existence of a local (peri-)sylvatic cycle of transmission. These results call attention to the potential introduction and subsequent spread in African urban areas of Asian genotype Zika virus currently circulating in the Americas and adapted to transmission by peri-domestic mosquitoes. They should leverage reinforced surveillance efforts in Africa.
The Asian genotype of Zika virus (ZIKV) has been responsible for recent outbreaks in the Pacific islands, the Caribbean and South/Central America where severe and formerly undescribed fetal and neurological complications of the disease,1,2 as well as non-vectored routes of transmission,3 have been observed. According to phylogenetic analyses, the Asian genotype of ZIKV emerged out of Africa ∼180 years ago.4 A striking observation is that the recent Asian genotype Pacific and New World circulating ZIKV strains are adapted to transmission by the vector Aedes aegypti5 and that this phenotypic trait is most probably crucial to understand their epidemic potential. By contrast, ZIKV strains belonging to the original African genotype have never been implicated in large outbreaks and the vector competence of African Aedes aegypti is low.6 Hence, there is considerable need for improving our knowledge about the ecology and epidemiology of the African genotype and in particular estimating the herd immunity of African populations against ZIKV. Although human cases of ZIKV infection have been reported in Africa since the early 1950s, this basic information remains essentially unavailable.
In this respect, 1084 blood donors from six sites of Cameroon representing a large panel of eco-environments were enrolled in a ZIKV seroepidemiology study from August to October 2015. They were administered an epidemiological questionnaire and serum samples were tested for the presence of IgG to ZIKV using the EuroImmun anti-NS1 IgG ELISA detection kit and a seroneutralization assay for confirmation of positives, as previously described.7 The observed global seroprevalence was low (∼5%), peaking at 10% and 7.7% in Douala and Bertoua, respectively, and as low as 2% in Maroua and Ngaoundéré (Fig. 1). In multivariate analysis, the most significant risk factors associated with ZIKV seropositivity were “to be a soldier” (p=0.021), a “high distance to the nearest house/shop” (p=0.042), and a “previous familial case of Yellow Fever” (p=0.001). Together with the low vector capacity of the African peri-domestic mosquito Aedes aegypti,6 these risk factors point to the existence of a (peri-)sylvatic cycle of transmission of ZIKV in Cameroon, similar to that of yellow fever virus, rather than to an urban “dengue-like” transmission of the virus.
Altogether, our findings indicate that the immunity of the Cameroonian population against ZIKV is low and that circulation in urban populations is uncommon. Hence, the risk of epidemic spread of ZIKV does exist. The epidemic emergence of the African genotype cannot be totally excluded but it would imply a first and uncertain step of adaptation of the virus to peri-domestic Aedes mosquitoes. More worrisome on the short term is the potential introduction in African urban areas of Asian genotype ZIKV currently circulating in the Americas, as previously suggested by modeling studies.8 The virus is likely to be imported by infected travelers coming from epidemic areas and has the potential to be transmitted by local peri-domestic mosquitoes. Our study provides biological evidence that such introduction would occur in populations that are globally immunologically naïve against ZIKV infection and live in areas where potential epidemic vectors exist.
This observation should lead to specific surveillance efforts and to a broader and more systematic mapping of at-risk populations in Africa. It also should ignite interest in investigating similar scientific and public health issues in the Asian population.
Conflicts of interestThe authors declare no conflicts of interest.
This work was partially supported by the European Union's Horizon 2020 Research and Innovation Program under ZIKAlliance Grant Agreement no. 734548.