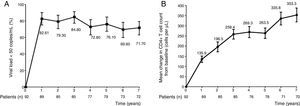
Management of highly antiretroviral therapy (ART)-experienced patients continues to be a challenge, and there are scarce “real-world” data about their long-term virologic and immunologic responses. Darunavir/ritonavir (DRV/r) was included among the antiretroviral drugs available in Brazil in 2008. Since then five Brazilian studies evaluated the effectiveness of salvage therapy, most of them DRV/r-based. In 2013, we published in this journal a single center prospective cohort study where 82.6% of patients achieved HIV RNA <50copies/mL after 48 weeks of treatment.1 Other four Brazilian retrospective observational studies were published showing 74.7–84% of patients with HIV RNA <50copies/mL. Follow-up in all these studies was also limited to 48 weeks.2–5 Herein, we report the results alongside seven years of our cohort in São Paulo. In line with the original report, non-completers were considered as failures in the analysis of virologic response. In 2016, seven years after initiation salvage therapy with DRV/r, 71.7% of patients had HIV RNA <50copies/mL and their CD4 cell counts increased by a mean of 353cells/mm3. Thirty-seven (40.2%) patients had CD4 cell counts above 500cells/mm3. Fig. 1 shows the annual results of viral loads and CD4 cell counts in this cohort. Despite the decrease in the rate of viral suppression after the third year of follow-up, high rates of viral suppression (about 70%) were still maintained afterwards. Interestingly, the increase of CD4 cell counts was steady and persistent throughout follow-up. Among patients on the 7th year of follow-up (n=72), 71 (98.6%) were receiving DRV/r, and 62 (86.1%) were given raltegravir in addition to DRV/r as part of their salvage regimen. Only 18 (25%) patients maintained the baseline regimen, 30 (41.7%) patients needed to discontinue at least one antiretroviral, and 32 (44.4%) patients needed to switch one or more components of their regimen. Our results showed that long-term benefit of ART is possible in middle-income countries, in spite of continuous and new challenges.
JEV has received honoraria for lectures and travel grants from Janssen-Cilag and Merck Sharp & Dohme. Other authors have no conflicts of interest.