The outcomes regarding portal hypertension-related complications and infections after HCV cure in decompensated cirrhosis are scarcely reported. We aimed to identify the predictors of survival and to evaluate the frequency of decompensation events of cirrhosis, including hepatocellular carcinoma (HCC), portal hypertension complications and infections in a cohort of decompensated cirrhotic with sustained virological response (SVR) in a real-world scenario.
Patients and methodsThis was a prospective study in consecutive HCV-infected patients with decompensated cirrhosis who achieved SVR after direct-acting antiviral (DAA) treatment. At baseline, clinical and laboratory data were recorded. Patients were followed until development of outcomes regarding further decompensation, death, or liver transplant. A Cox-regression analysis was performed and survival curves were constructed using the Kaplan Mayer method.
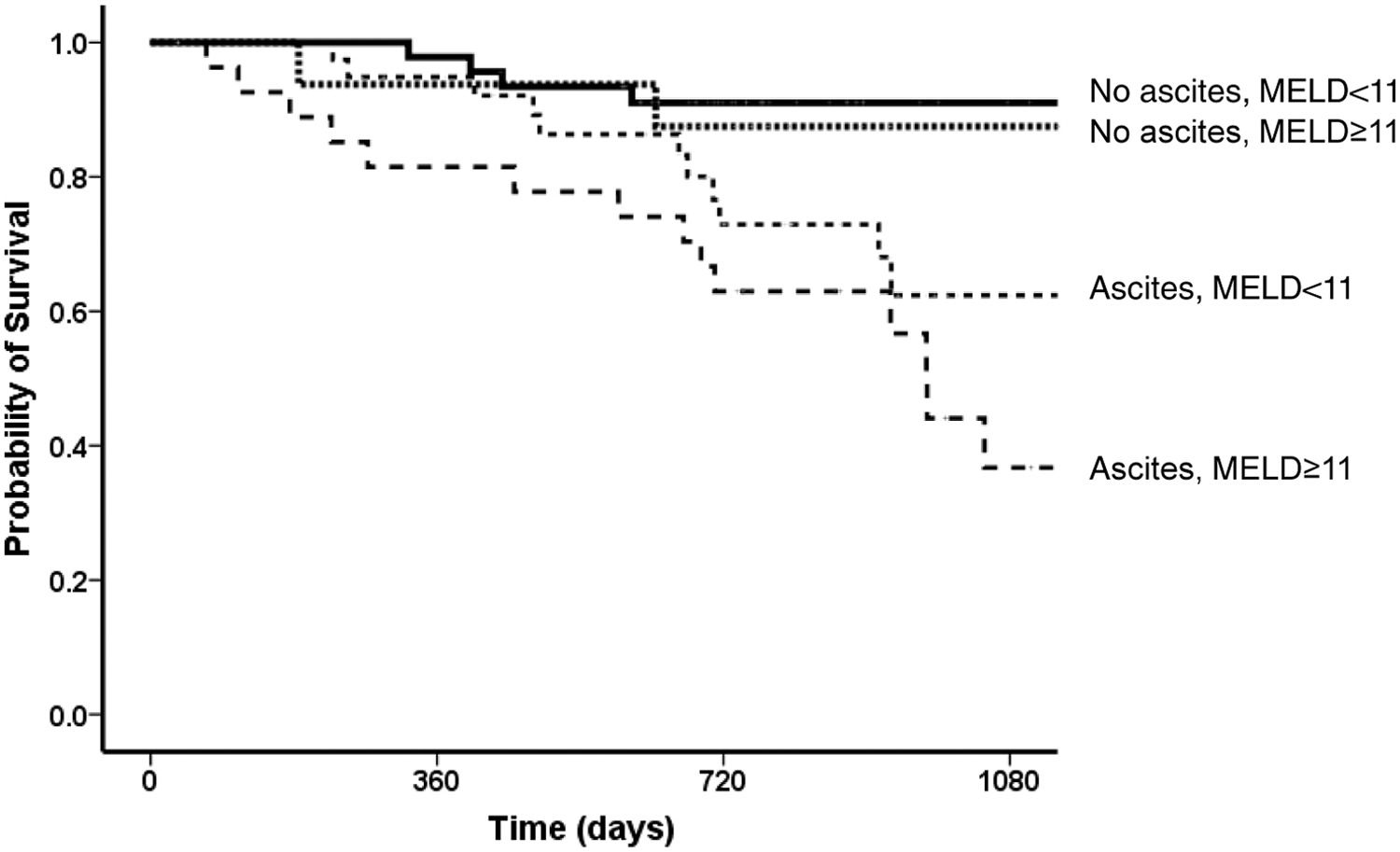
ResultsOne hundred and thirty patients (age 60 ± 9 years, 64% female, 70% genotype 1) were included and followed-up through three years. SVR was associated with a lower prevalence of ascites and an improvement in Child-Pugh and MELD scores. One and three-year probability of transplant-free survival was 93% and 66%, respectively. Variables related to three-years survival were MELD < 11 (HR 1.24, 95% CI 1.13-1.37) and absence of ascites (HR 2.03, 95% CI 0.99-4.13) after the end of treatment (91% versus 37% in patients with ascites and a higher MELD, p < 0.001).
ConclusionsDecompensated cirrhotics with SVR and a low MELD without ascites have an excellent long-term prognosis. On the contrary, those with higher MELD and ascites have a low probability of survival even in the short term and might be evaluated for liver transplantation.
Direct Acting Agents (DAA) treatment for chronic hepatitis C (CHC) achieved higher rates of sustained virologic response (SVR) with shorter treatment duration, fewer adverse events and better patient tolerance.1 These findings were replicated even in populations previously considered as difficult to treat, like those with cirrhosis.2
Despite these encouraging data, some concern persists regarding the treatment of decompensated cirrhotic patients, such as a slightly higher rate of adverse events and drug interruption and lower SVR rates.3-5 In decompensated cirrhotic patients SVR is associated with early, albeit modest, improvement in liver function scores like MELD.6 These changes, however, may not translate into clinical benefit, as these individuals, with a decrease in MELD score, may be less likely to receive liver transplantation.7-9 Of note, there are few well-established pre- or post-treatment parameters capable of identifying patients more likely to show transplant-free survival in the long term after SVR10 and it has not been established if pre- or post-SVR parameters would better predict long-term outcomes.
Determining the frequency and timing of these complications may be of great value for either instituting or withdrawing screening and prophylactic measures. Long-term follow-up of these individuals would better clarify these issues. However, most studies have followed these patients for short periods, less than two years.11,12
The aim of the present study was to determine the frequency of long-term complications of cirrhosis and survival after SVR on a cohort of decompensated cirrhotics treated in a real-world scenario with DAA. Besides, the study aimed to describe pre- and post-antiviral treatment predictors of transplant-free survival.
MethodsA prospective study was carried out in two tertiary hospitals in Rio de Janeiro.5 Consecutive HCV-infected decompensated cirrhotics treated with DAA from 2015 to 2017 were included and followed until liver transplantation or death. The IRBs and ethics committee from both centers approved the study. All patients provided written informed consent. Inclusion criteria were age ≥ 18 years old, SVR after DAA treatment, and diagnosis of current or previous decompensated HCV-related cirrhosis.
Cirrhosis was defined according to previously established criteria.13,14 A Child-Pugh score ≥ B7 and/or a MELD score ≥14 at baseline was defined as decompensated cirrhosis.15 Previous decompensation was defined as patients with Child-Pugh score A or MELD lower than 14 who were under treatment for cirrhosis-related complications. Exclusion criteria: presence of other concomitant etiologies for chronic liver disease, HIV infection, previous liver transplantation, hepatocellular carcinoma, pregnancy, severe cardiorespiratory disease, renal failure requiring hemodialysis and failure to achieve SVR.
At baseline and in the period between the end of treatment (EOT) and evaluation of SVR, clinical and laboratory data were recorded, including age, sex, mean arterial pressure, heart rate, weight, clinical co-morbidities, presence of ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, portal-hypertension related gastrointestinal bleeding and the prescribed DAA regimen, and HCV genotype at baseline. Child-Pugh and MELD scores were also calculated. After SVR, patients were maintained on regular follow-up screening for hepatocellular carcinoma and esophageal varices at regular intervals according to international recommendations.16,17
Development of new or worsening of previous complications of cirrhosis was recorded and treated following current clinical practice guidelines at the time of the study.18 According to the site and origin of infection, treatment of bacterial infections followed institutional recommendations and international guidelines.19
Statistical analysisCategorical variables were described as frequency and percentage and continuous variables as mean and standard deviation or median and interquartile range (IQR). For a contrast of hypothesis, Chi-square and Student's t-test were used for categorical and continuous variables, respectively. ROC curves were used for the selection of the best cut-off points for Child-Pugh and MELD scores related to survival. Baseline and post-SVR factors associated with mortality were selected for multivariate analysis using Cox-regression models (backward stepwise selection method). For the Cox regression analysis, variables with a p-value < 0.1 at univariate analysis were included in the survival model. In order to establish the best cut-off for MELD and Child-Pugh (initially continuous variables), we performed ROC curve analysis using c-Statistics. Based on the best points of sensitivity and specificity in the AUROC for survival, we included these variables as categorical ones for the survival analysis. Survival curves were constructed using the Kaplan Mayer method, and comparisons were performed using a log-rank test. Patients were censored at the time of transplantation or death for assessment of survival purposes. Two-tailed p-values < 0.05 were accepted as statistically significant. All analyses were performed using the IBM SPSS 21 for Windows.
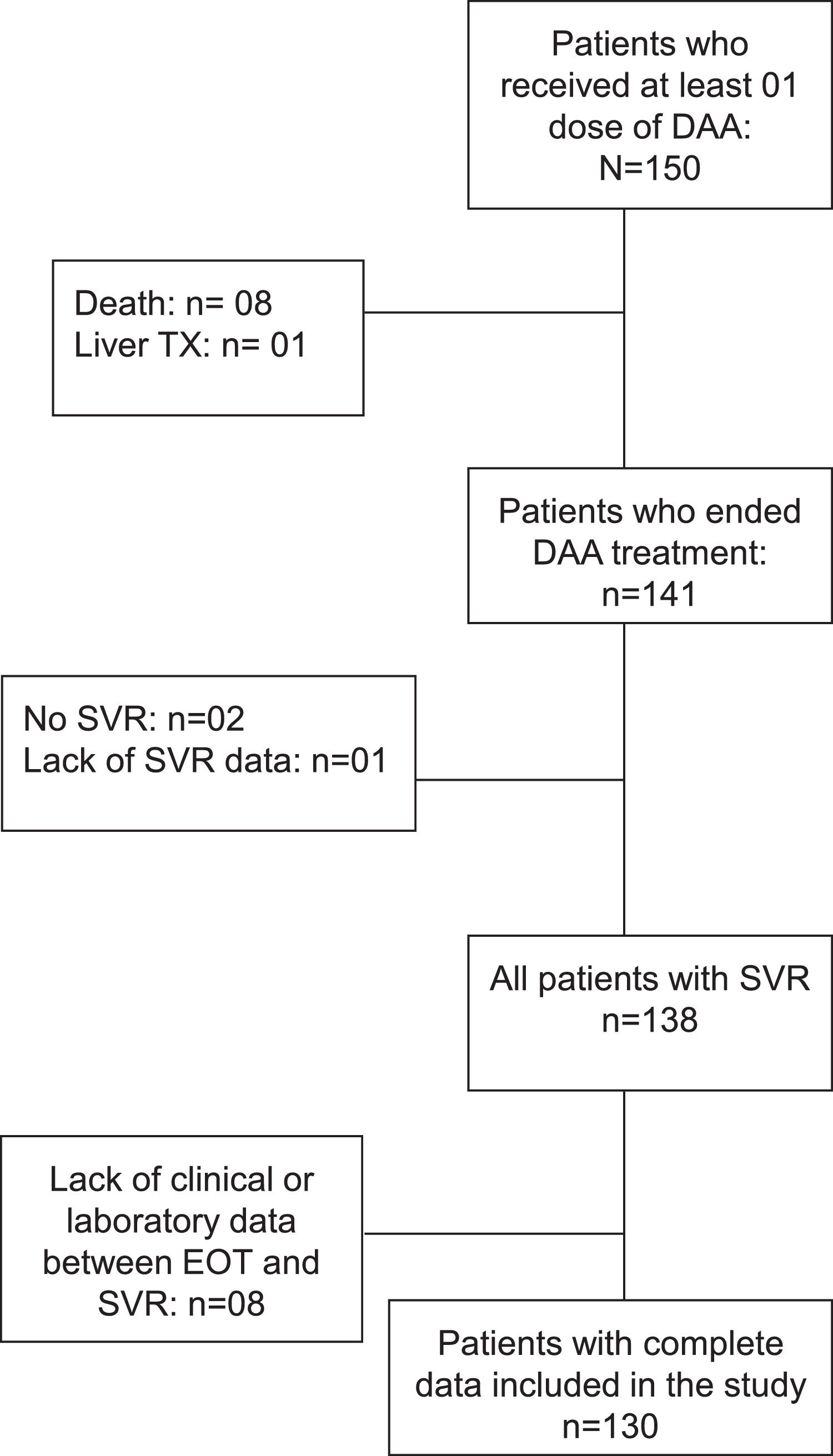
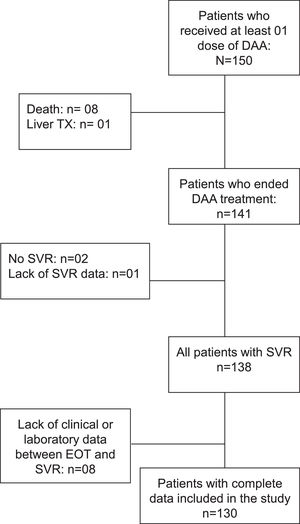
ResultsAt baseline, 150 patients have received at least one dose of DAA. Eight patients died and one underwent liver transplantation before the end of treatment. Among the 141 patients who ended treatment, one was excluded due to lack of data regarding SVR, two for not achieving SVR, and eight did due to unavailability of data between the end of treatment (EOT) and SVR. Hence, 130 patients were ultimately included (Fig. 1); 64% female, age 60 ± 9 years, 39% had type 2 diabetes mellitus (T2DM), 46% systemic arterial hypertension (SAH). Genotype 1 was the most prevalent (42% genotype 1a, 28% genotype 1b, and 15% without sub-genotyping) and 12% were genotype 3. Ninety-four patients (72%) received treatment with Sofosbuvir, Daclatasvir, and Ribavirin, and 36 (28%) received Sofosbuvir plus Daclatasvir. The comparative analysis between baseline and SVR characteristics are shown in Table 1.
aseline and 12-week post-end-of-treatment laboratory parameters and liver function-related complications of patients with decompensated cirrhosis treated with DAA (n = 130).
DAA, direct acting antivirals; EOT, end of treatment; BUN, blood urea nitrogen.
SVR was associated with improvement in many clinical and laboratory parameters (Table 1). Concerning Child-Pugh score and MELD scores, 8% Child-Pugh score class A patients became class B/C and 45% of Child-Pugh score class B/C improved to class A. The remaining patients did not change the Child-Pugh score. The median change in MELD score from baseline to SVR was -1 (IQR -2 to +1); in 67% of patients MELD score did not vary more than two points. MELD score improved three or more points in 22% of patients, and in 10% it worsened by more than three points.
Overall, the median time of follow-up was 756 (IQR 637-978) days after EOT.
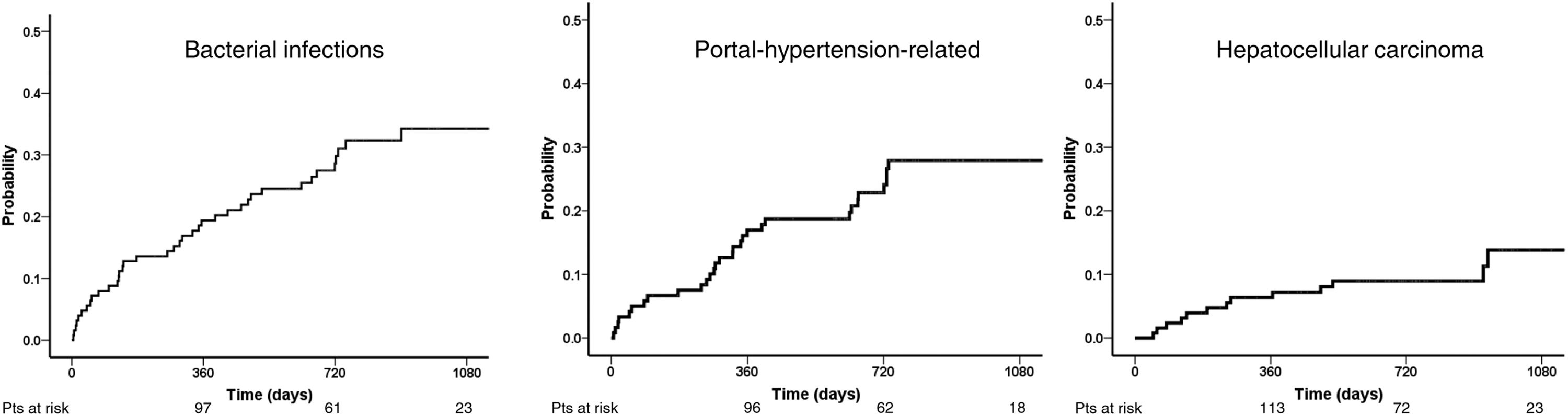
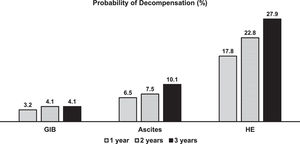
Forty patients (31%) had at least one bacterial infection after a median of 270 (52-488) days. One-, two- and 3-year probability of infection was 19.4%, 28.6%, and 34.3% (Fig. 2). The most common infection was pneumonia, followed by urinary tract infection and cellulitis (11, 7, and 5 patients, respectively). Bacterial infections were more frequent in patients who developed portal-hypertension related complications (53% vs. 24%, p = 0.003) but not in those with HCC (31 vs 32%, p = 0.95). Bacterial infections were associated with increased probability of mortality (12.5%, 31.3%, and 66.6% at 1, 2, and 3 years versus 2.3%, 14.3%, and 27.2% for those who did not, p = 0.018).
Thirteen patients (10%) developed hepatocellular carcinoma after a median of eight (IQR 3-17) months after EOT. The probability of development of HCC at 1, 2, and 3 years was 7.2, 8.9, and 13.7% (Fig. 2). Patients with MELD equal to or above 11 showed a higher incidence of HCC compared to those with MELD under 11 (77% vs. 23%; p = 0.04). Nevertheless, there was no significant difference in the proportion of HCC between Child-Pugh score classes B/C vs. Child-Pugh score class A patients (54% vs. 46%; p = 0.47).
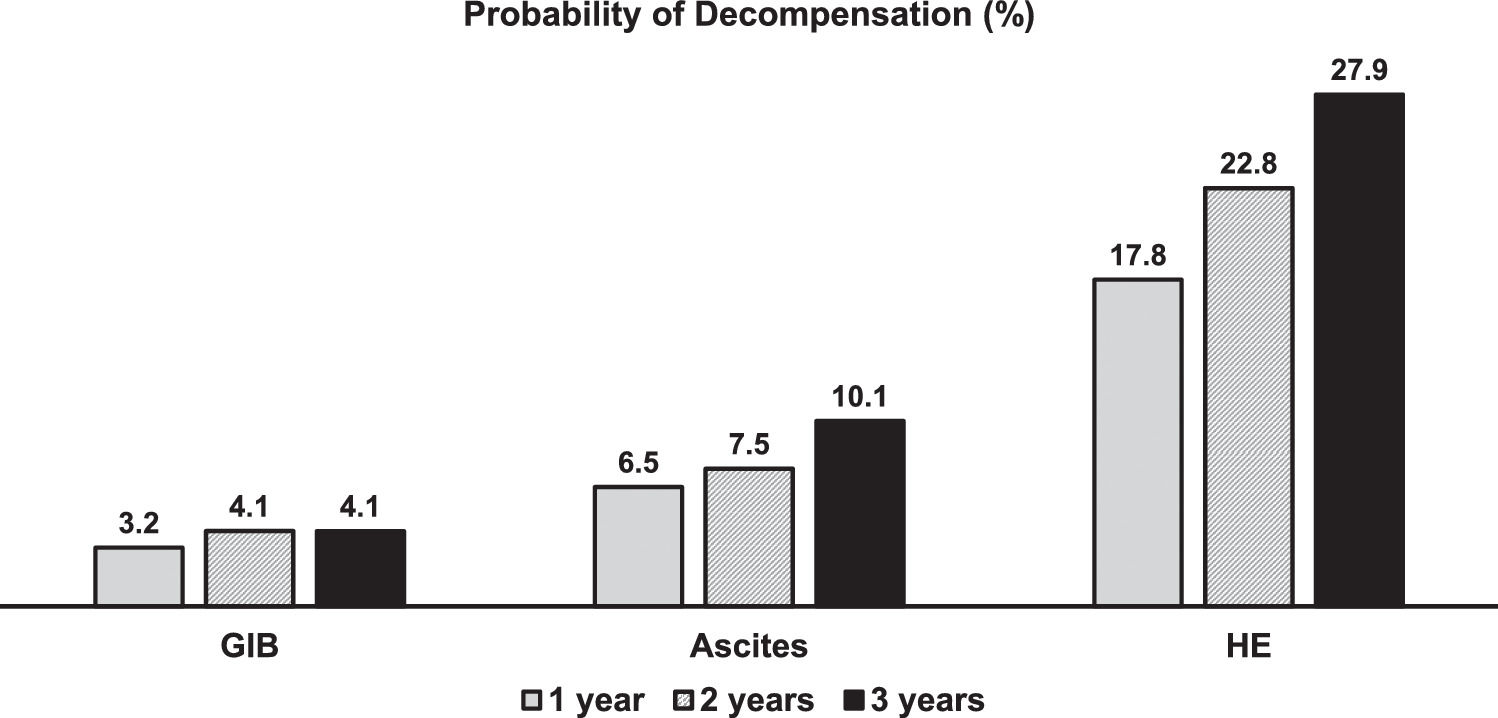
Thirty patients (23%) developed at least one complication of cirrhosis related to portal hypertension during follow-up. The median time for developing the first complication was 304 days (IQR 94-631 days). One-, 2- and 3-year probability of portal-hypertension-related decompensation was 17.0, 22.8, and 27.9% (Fig. 2). Fig. 3 shows the probability of each portal hypertension-related events during 3-years of follow-up. Hepatic encephalopathy was the most frequent complication observed in 21 patients, followed by ascites and varicose gastrointestinal bleeding (11 and 5 patients, respectively). Development of more than one complication was unusual, observed only in four patients (hepatic encephalopathy in all of them, associated with ascites and varicose gastrointestinal bleeding in two patients each). The development of portal-hypertension-related complications did not impact survival (93.3%, 75.1%, and 60.1% at 1, 2 and 3 years versus 96.7%, 85.3%, and 70.7%, p = 0.11).
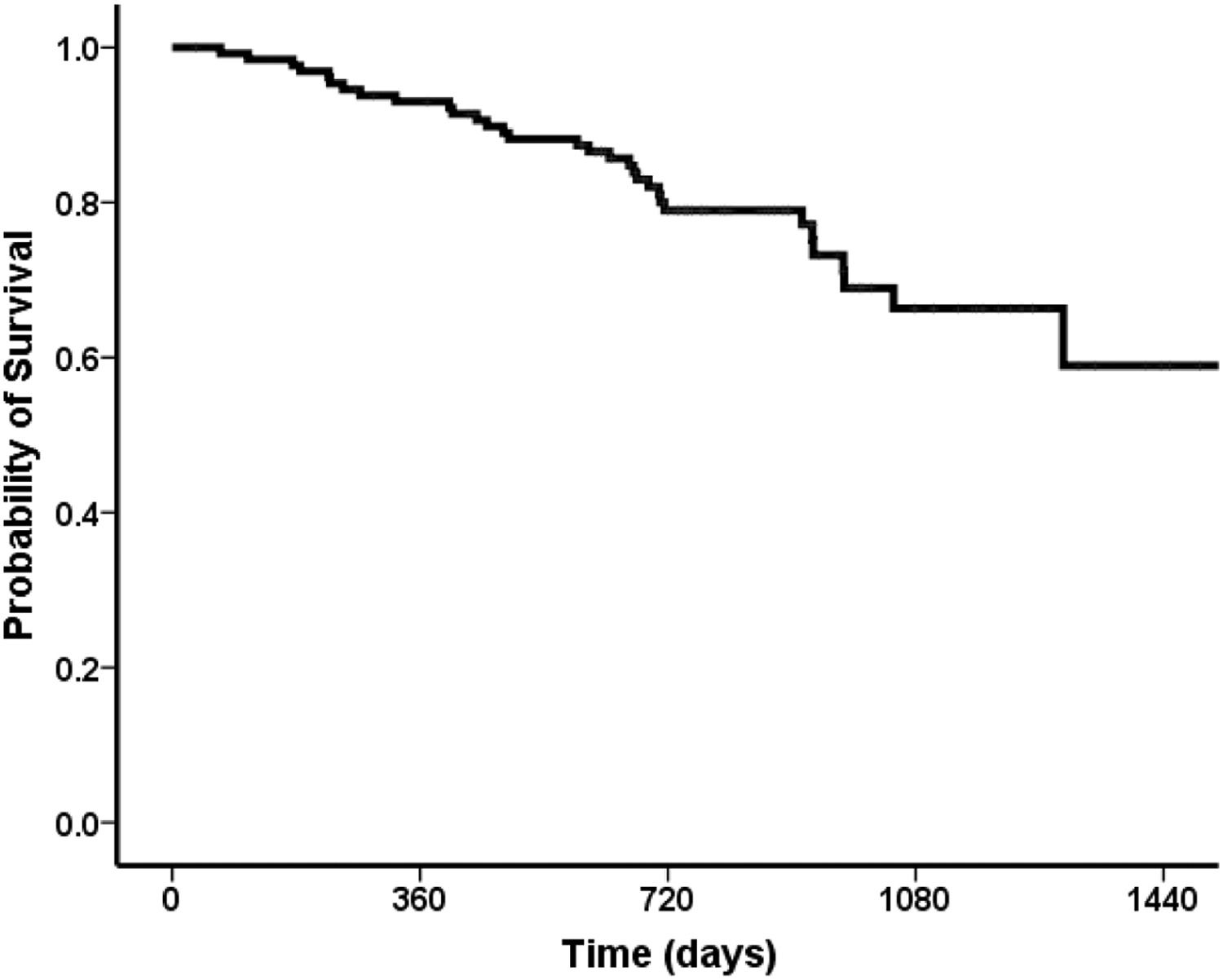
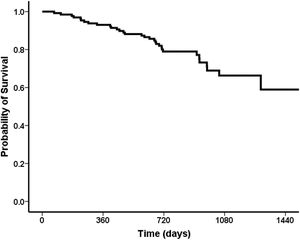
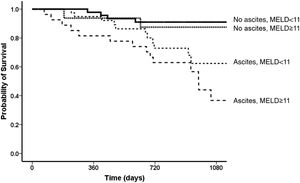
Overall, 91 (70%) patients were still alive at the end of follow-up, 26 (20%) patients died during follow-up, six patients (4%) were submitted to liver transplantation, and seven (6%) were lost to follow up. The probability of survival at one year after the end-of-treatment was 93%, followed by 80% in two years, 66% in three years (Fig. 4).
In univariate analysis, the factors related to survival were Child-Pugh and MELD scores, AST, albumin and bilirubin levels, and absence of ascites after the end of treatment. The best cut-offs for Child-Pugh score and MELD after the end of treatment related to survival, were eight for Child-Pugh score (AUROC = 0.67 CI 95% 0.55-0.78; p = 0.004) and 11 for MELD score (AUROC= 0.72 CI 95% 0.61-0.83; p < 0.01), respectively. In the multivariate model, the independent variables associated with survival were a MELD score under 11 in the post-treatment period (HR 1.24, 95% CI 1.13-1.37, p < 0.001) and absence of ascites at the same period (HR 2.03, 95% CI 0.99-4.13, p = 0.053) [Fig. 5]. Considering both variables (ascites and MELD), the best survival was observed for those without ascites who had a MELD score < 11 (one-year survival = 98%; three-year survival = 91%) when compared to those with ascites and a MELD score ≥ of 11 (one-year survival = 82%, three-year survival = 37%; p < 0.001). For patients without ascites and a MELD score ≥ of 11, the one-year survival was 94% and three-year 88%, and for those with ascites and a MELD score <11, the one-year survival was 95% and three-year 62% (Fig. 4).
DiscussionThis study prospectively evaluated DAA-treated decompensated patients with cirrhosis followed for a period of up to three years. It has three main findings. First, the best prognostic factors related to survival were MELD score and absence of ascites at the end of treatment. Patients with a MELD score lower than 11 and without ascites up to 12 weeks after treatment had a three-year probability of survival higher than 90%. Second, although patients are prone to develop complications after SVR, neither portal-hypertension-related complications nor HCC have impacted survival. Third, 66% of patients were alive and free of liver transplant at the end of a follow-up of three years.
Most longitudinal studies that assessed HCV-infected long-term outcomes evaluated only the impact of SVR on mortality and development of HCC and in general, included both compensate and decompensated cirrhotics.20 The long-term impact of HCV treatment in decompensated cirrhosis is still scarce,21,22 so the decision to treat this population is not easy. In SVR decompensated cirrhotics, liver function is usually improved within six months. It has been demonstrated that some patients present significant changes of Child-Pugh and MELD scores in the period between the end of treatment and SVR that may have prognostic relevance. In the present study, liver function-associated variables like bilirubin and albumin levels, but not those related to portal hypertension as platelet count, presented a substantial improvement after SVR. Of note, patients with ascites before treatment significantly improved in the short-term follow-up.
El-Sherif et al. have retrospectively performed a pooled analysis comprising 502 Child-Pugh B and 120 Child-Pugh C patients in four clinical trials with Sofosbuvir-based regimens.23 Ascites or encephalopathy at baseline, serum level of albumin below 3.5 g/dL, ALT under 60 U/L, and body mass index (BMI) above 25 kg/m2 were associated with lack of improvement in Child-Pugh score or maintenance of patients in the “purgatory of MELD”, independent of SVR. In our cohort, we investigated the outcome of decompensated cirrhotic patients who had already attained SVR. We observed that 45% of Child-Pugh B/C improved to Child-Pugh A, and only 8% of Child-Pugh A patients became Child-Pugh B/C.23
However, we observed that the presence of ascites together with a MELD score higher than 11 independently and negatively impacted survival. This finding is helpful since these variables should draw attention when evaluating these patients post-treatment. In our cohort, the survival rate of patients who presented ascites and a MELD equal to or higher than 11 was lower than 40% on a three-year follow-up. In the retrospective study of Carrillo et al. in compensated and decompensated cirrhotic patients treated with DAA, a MELD score above 18 was an independent risk factor for complications during and 12 weeks after treatment.12 In our study, the worst scenario was a composite of MELD equal to or above 11 plus ascites. According to the study of Carrillo et al., a MELD above 11 alone did not impact survival.
Verna et al. followed cirrhotic patients from the TARGET cohort for up to four years. Similarly to our study, the median baseline MELD was 12, 7% of patients died, and 3% had a liver transplant. Our study included a similar proportion of Child-Pugh score A patients with a previous episode of decompensation, who had a poor prognosis in this group independently of Child-Pugh or MELD score.24,25 However, we included more Child-Pugh B patients (74% vs. 48%)(10). There was an important improvement in Child-Pugh score on a short-term basis, since 45% of Child-Pugh B/C improved to Child-Pugh A between EOT and 12 weeks after EOT. Regarding MELD improvement, our results are similar to Verna et al.10 These results highlight the impact of improving CHILD score and MELD even in a real-world scenario. Pereira Guedes et al. evaluated 237 HCV-infected patients in a retrospective study.26 Only 3.1% were Child-Pugh score class B, and 16.9% had presented hepatic decompensation before treatment. The liver-related mortality was 2.2% during 27 months of follow-up. In our study, 93%, 80%, and 66% were alive by the end of the first, second, and third year, respectively. This difference is probably related to a higher proportion of Child-Pugh score B/C patients in our study.
Despite the consistent improvement in liver function parameters, the development of further decompensations of cirrhosis was frequent in our cohort. Almost 25% of patients developed portal hypertension-related complications in the post-treatment period. The most common complication was hepatic encephalopathy, followed by ascites, and variceal bleeding. These numbers differ from those previously reported by Pereira-Guedes.26
Such a high frequency of portal-hypertension-related decompensation may not be entirely unexpected. A previous study demonstrated the persistence of portal hypertension in cirrhotic patients shortly after SVR.27 Remarkably, all liver-related complications such as hepatic encephalopathy, HCC, and infections were more frequent in the short-term follow-up before one year. Notably, infections kept increasing along with the follow-up while portal-hypertension-related events became stable.
We observed a higher incidence of HCC (10%) early in the first year of follow-up, compared to Pereira-Guedes et al.26 Maybe this might be due to the higher number of patients with more advanced disease in our study. A higher proportion of T2DM patients (39%) might have also impacted the increased risk of HCC.28 Nahon et al. have shown that metabolic risks increase the odds of HCC29 and Mecci et al.30 observed that the diagnosis of diabetes increased the likelihood of HCC in HCV-related advanced liver disease. Calvaruso et al. described a 7.8% rate of HCC development among Child B patients who achieved SVR, most of whom also developed HCC in the first year after SVR.31 In the study by Verna et al., 16 patients developed HCC, three had a MELD higher than 16.10 On the other hand, in our study, patients with a higher MELD had a higher incidence of HCC, although it was not statistically significant. There was also no difference in the Child-Pugh score amongst patients who developed HCC. We noted that the incidence of HCC remained stable over the follow-up period. Hence, these patients must be kept on careful screening every six months.
Bacterial infections were quite frequent in our population. Few studies have discussed the impact of infections on the follow-up of patients.10 In our study, patients had an increased risk of bacterial infections until the end of the second year post SVR. Also, development of infections was particularly common in patients with portal-hypertension-related decompensation. Most studies in decompensated cirrhotic patients did not follow patients long eough; maybe this was the reason for not identifying infection in this population.
The main limitation of this bicentric study is the number of patients included in the cohort, which, compared to other studies, is relatively small. However, unlike other published studies, our cohort comprises decompensated Child-Pugh score B/C cirrhotic patients with a long-term follow-up.
In conclusion, DAA-treated decompensated HCV-cirrhotics present a post-SVR clinical course with systematic development of cirrhosis-related complications, a high incidence of hepatocellular carcinoma, and bacterial infections. However, a MELD score lower than 11 and the absence of ascites is an excellent prognostic composite variable, mainly in the first two years after SVR. On the contrary, those with higher MELD and ascites have a low probability of survival even in the short term and should be evaluated early for liver transplantation.
Gustavo H. Pereira received funding from Estácio de Sá University (Programa Pesquisa e Produtividade UNESA). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.Cristiane A.Villela-Nogueira and Renata M. Perez receive research grants from Brazilian National Research Council for Scientific Development and Technology (CNPq) and FAPERJ – Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Brazil. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.