Leprosy is an infectious disease caused by Mycobacterium leprae. This study aimed to understand the distribution and impact of Hansen's disease in different regions of Brazil and the outcome of cases in recent years.
MethodologyAn ecological study was conducted throughout the Brazilian territory for 11 years (2008–2018). The mortality rates, new cases of the disease, and proportion of physical disability and cure were assessed. Data were obtained using DATASUS. GraphPad Excel and “Prisma” programs were used for tabulation and data processing. To present an accurate perspective from all regions, the data were normalized according to the population.
ResultsOverall, the results demonstrated a significant reduction in the number of leprosy cases in Brazil over the last decade (p < 0.05). North and Midwest regions showed higher mortality rates standardized by age (p < 0.05). The largest number of cases were found in Tocantins and Mato Grosso, inner states of Brazil (p < 0.05). The cure rates in the Southeast and South regions were negatively correlated with the mortality rate in these regions (p < 0.05).
ConclusionsActions to control the spread and unfavorable outcomes of leprosy have been effective in the last decade in most Brazilian states. On the other hand, the states of Tocantins and Mato Grosso, in the North and Midwest regions of Brazil, need to intensify the fight against the disease. Notwithstanding, measures against leprosy should continue and be intensified in regions with greater aggravations, aiming at an effective homogeneous control of the disease.
Hansen's disease is an infectious illness caused by Mycobacterium leprae)1 which develops slowly and can have an incubation period of 2–7 years.2 This Gram-negative bacterium infects susceptible individuals, preferentially affecting the skin and peripheral nerves, causing loss of tactile sensitivity and motor disability.1
M. leprae is an intracellular acid alcohol-resistant bacillus that infects the cells present in the nervous system and epithelial tissue. M. leprae bacillus can be found in keratinocytes, macrophages, and neutrophils and affects Schwann cells within peripheral nerves.3 Keratinocytes may play an important role in the activation of the antimicrobial peptide beta-defensin, which acts in response to the bacillary antigen. When M. leprae enters the host cell, it triggers a metabolic lipid interaction in order to survive.4-6 Thus, after cell infection, an appropriate environment for bacterial multiplication is induced.7 This leads to dermatological manifestations and infectious nerve involvement which subsequently causes axonal alterations and demyelination, causing sensory damage, disability, and deformity.5,8,9
Leprosy can present itself in different ways, depending on the immune response of the infected individual. Therefore, it is necessary to use the Ridley-Jopling classification, which subdivided the syndrome into five types: polar tuberculoid leprosy (TT), borderline tuberculoid leprosy (BT), borderline–borderline leprosy (BB), borderline lepromatous leprosy (BL), and polar lepromatous leprosy (LL).10–13
There is also the less complex WHO classification system based on the number of associated skin lesions: up to five lesions are considered as paucibacillary form, and from six lesions as multibacillary leprosy.14
Clinically, the disease presents itself mainly through the skin lesions and sensory changes, although visceral involvement (such as eyes, testicles, and bones) may be present. Tuberculoid forms are generally characterized by the appearance of hypochromic macules with a hyposensitive component surrounding the lesion. On the other hand, the lepromatous form is generally more aggressive, with a large number of bilateral and asymmetric erythematous lesions (mainly on the face, auricular lobe, and fingers), in addition to bilateral, symmetrical, and diffuse peripheral neuronal involvement, which may be associated with hypertrophy of the affected nerve, as well as sensory and motor deficits.10–12
M. leprae can affect individuals of all ages and is less common in children. The infection is equally prevalent in both sexes. Transmission occurs through direct contact between an individual with an infection and household contacts.4
M. leprae transmission pathways are not fully understood, but several studies suggest that this bacillus is preferentially dispersed by the nose, instead of the skin.15–17 Genetically defined immunological mechanisms related to human leukocyte antigens (HLA) play an important role in the proliferation or destruction of bacilli and consequently in resistance to infection.18 The risk of developing leprosy is also associated with the endemic level, adverse socioeconomic conditions, and social and health precariousness, which explains a large number of cases in underdeveloped countries.19
Public health indicators can be an essential tool for predicting the behavior of this disease. Although there is currently a wide range of drug therapies that effectively cure Hansen's disease, the main challenges lie in monitoring new cases as well as the patient's adherence to the prescribed treatment.5
The increased effectiveness of therapeutic regimens that emerged in the 1980s enabled a significant reduction in the global prevalence of leprosy. In 1985, there were about 5.3 million cases and by the end of 2017 this number had dropped to 192,713 cases.20,21 However, in the last decade there was an increase of new cases: 210,758 in 2015, 214,783 in 2016, and 210,000 in 2017 in 150 countries, with a notification rate of 2.77 per 100,000 inhabitants.20,22 Prospective assessments of the disease are difficult to understand because of the ongoing changes proposed in its definition, its long incubation period, and changes in its control activities causing delay in case detection.23 Furthermore, as the surveillance systems of low-income countries are supposedly affected by significant inaccuracies, even in endemic areas, the available numbers are affected by a significant underreporting.21,24 In addition to these difficulties social stigma, untrained health professionals further compromise the appropriate patient assessment.25 In the Northeast region of Brazil, 7067 hospitalizations were reported with 147 deaths and a mortality rate of 2.08% between the period 2012 and 2017.26 Therefore, there is a need for extensive research to improve the understanding of leprosy in its various aspects including its epidemiology.
According to the United Nations, Brazil accounts for 11.6% of all leprosy cases worldwide. To modify these figures, the Brazilian Unified Health System operates through programs aimed at both treatment and prevention of this disease. The period between 1995-2000 marked a milestone in Hansen's disease in Brazil, with the implementation of measures outlined by the leprosy elimination plan (PEL). To increase resolution, this contingency plan prioritized its implementation at the municipal level so that in 2006 the plan was fully implemented at that level. Thus, there was better management and coordination in the fight against leprosy at the primary health level aiming to establish effective monitoring of this infection.27,28 Over the past decade, other initiatives at the municipal, state, and federal levels have been maintained or implemented. Understanding the outcome of these efforts is essential to outline combat strategies in the near future. Thus, this study aimed to evaluate the mortality rate due to the disease, as well as number of new cases, and the rates of physical disability and cure in the Federative Units of Brazil.
MethodologyEthical considerationsAs this research was based on public data from secondary source, ethical approval to carry out the study was not necessary, but met the current principles of resolution number 466 from the National Council of Health of 2012. The data do not have personal identifiers of the cases, containing only information concerning public health.
Study siteData were collected at the Department of Informatics of the Unified Health System (DATASUS) of the the Brazilian Ministry of Health on the website http://www.datasus.gov.br, accessed in October/November 2020. The target of this study was all notifications of new cases and deaths from leprosy in the period from 2008 to 2018 that occurred in Brazil. To better assess the country data, strata by macro-regions (North, Northeast, Southeast, South and Midwest) and states present in each macro-region (27 federative units) were considered.
Type of study and data extractionAn ecological study was conducted using a database. The DATASUS database was used to obtain data on the number of deaths due to leprosy that occurred in Brazil from 2008 to 2018. In addition, data on notifications of the disease in the period 2015 to 2018 were obtained for municipalities in regions with the highest prevalence of leprosy. The specific mortality rate was standardized by age using the direct method, considering the standard population in Brazil for each year of the study. The population projections of Brazil and of each Federation Unit by sex and age were collected from the Brazilian Institute of Geography and Statistics website (IBGE).
Mortality rates due to Hansen's disease in the Brazilian states were compared, as well as trends in leprosy death rates. The relation between number of new cases of leprosy and morbidity and mortality was evaluated. In addition, the distribution of leprosy cases, rates of physical capacity loss due to the diagnosis and of cure were also investigated.
Elegibility criteriaAll deaths from leprosy registered at the DATASUS platform, from 2008 to 2018, were included.
Statistical analyzeThe data were tabulated using Excel (Microsoft Office Professional Plus, 2016). Statistical analyses were performed using GraphPad Prism program 8.0 of Graphpad (http://www.graphpad.com). To display the data graphically, the QGIS 3.10 program (https://qgis.org/en/site/) was used, with the cartographic bases of the Brazilian Institute of Geography and Statistics, 2017. Normality was tested for all variables (D'Agostino & Pearson or Shapiro-Wilk test). Nonparametric tests were applied to compare the groups (“Kruskal-Wallis statistic” with “Dunn's Multiple Comparison” post-test) and the data were correlated using the Pearson and Spearman test.
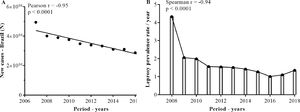
ResultsProfile and distribution of new leprosy cases among Brazilian statesAs shown in Fig. 1, over the years, there has been a statistically significant decline in the number of new cases and the prevalence of leprosy per year in Brazil (Fig. 1A and B).
Distribution of new cases and prevalence of leprosy in Brazil from 2008 to 2018. Data were obtained from the digital database of the Ministry of Health (DATASUS), from 2008 to 2018. The data were normalized for the number of occurrences per 100,000 inhabitants, according to information on population density according to the IBGE. Pearson and Spearman's tests were used to compare the different groups.
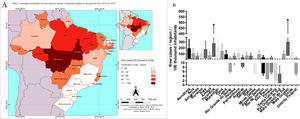
The correlations between the incidence rates in the Brazilian states in relation to the period of the study (2013 to 2017) showed two Brazilian states, namely Mato Grosso and Tocantins, with significantly higher correlations. Most of the new cases of leprosy in the Midwest were in the state of Mato Grosso where there was a higher number of new cases per 100,000 inhabitants compared to other states of the federation. Tocantins, located in the Northern region, also showed prominence in the rate of new cases per 100,000 inhabitants (Map 1). There were no statistically significant differences among the other Brazilian states in terms of number of new cases per 100,000 inhabitants in the evaluated period (Fig. 2).
Distribution of new cases of leprosy in the Brazilian states per 100,000 inhabitants (2013 to 2017). The data were normalized for the number of occurrences per 100,000 inhabitants, according to information on population density according to the IBGE. In A, the mean distribution of new leprosy cases can be visualized for the different Brazilian states on the map. In B, the distribution of new leprosy cases by interquartile range for each Brazilian state.
During the period evaluated (2008 to 2018), 1,801 deaths were recorded in Brazil, with 44% occurring in the Northeast, followed by the Southeast (21%), Midwest (15%), North (14%), and South (6%). The leprosy mortality rates standardized by age per 100,000 inhabitants, specifically in the different macro-regions, considering possible temporal correlations (2008 to 2018) showed a significant negative correlation (p < 0.05) between deaths in the Northeast (r = -0.90), Southeast (r = -0.72), and South (r = -0.66) regions of Brazil. In the North (r = -0.51) and Midwest (r = -0.39) regions, no significant correlation was found (p > 0.05) (Fig. 3A). When the mortality rates standardized by age per 100,000 inhabitants during the studied period were compared among regions, the Southeast and South regions had lower rates than other regions (North, Northeast, and Midwest) [p < 0.05]. On the other hand, there were no significant differences among North, Northeast, and Midwest regions. The lower value of the 95% confidence interval of the mortality rates in North and Midwest regions were higher than the upper limit for Brazil as whole (Fig. 3B).
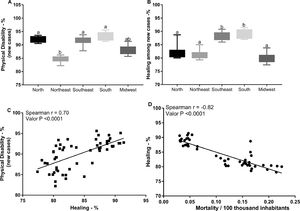
Association between the number of new leprosy cases and the rates of physical disability, cure, and mortality in Brazilian statesIn addition to the mortality prevalence data, we verified the possible relationships between the number of new cases of leprosy in the Federative Units of Brazil and the rates of physical disability and cure. The Southeast and South regions showed higher rates of physical disability and cure in relation to the other macro-regions (p < 0.05), whereas the death rates were higher in the North, Midwest, and Northeast regions (Fig. 4A and B). In addition, positive (r = 0.70) and significant (p < 0.0001) correlations were observed between the rate of physical disability in new cases and the rate of cure. In contrast, the frequency of cure rate was negatively correlated with the disease mortality rate (r = -0.82, p < 0.0001) (p < 0.0001) (Fig. 4C and D).
DiscussionLeprosy diagnosis can be delayed by multiple factors. There is still a lot of prejudice surrounding the disease. Thus, infected people tend to hide their diagnosis from household contacts, who should also be monitored and receive adequate attention. Furthermore, it is difficult to diagnose the disease, and this leads to postponement of treatment, which implies in higher grades of disease complications and even death.
In the present study, it was possible to find new cases and deaths in various parts of the country and identify a significant reduction in the number of leprosy cases in Brazil in the last decade. Furthermore, there were discrepancies in the distributions of notifications and mortality in the different macro-regions of Brazil. The North and Midwest regions had higher mortality rates. The largest number of cases was found in Tocantins and Mato Grosso, states in inner Brazil. Finally, we report that the cure rate in the southeast and south regions were negatively correlated with the mortality rate in these regions.
To overcome these problems, many efforts have been made over the years, and understanding the effectiveness of these efforts is vital. Epidemiological studies contribute significantly to understanding the disease and its implications and serve indirectly to verify whether public health policies minimize the damage caused by the disease as well as the costs for the state.
The reductions in new cases and mortality, observed in the study, are presumably due to proper implementation of the Leprosy Control Program in primary care nationwide. From an epidemiological perspective, such a result points to a tendency of low prevalence or even stabilization of the endemic.18 In addition, the leprosy prevalence rate per year in the analyzed period showed a decreasing trend, as well as stability. On the other hand, Brazil is still one of the countries with the highest prevalence of leprosy cases worldwide and has not managed to reach the WHO goal of one case per 10,000 inhabitants.28
Herein, we show the variation in death rates among the Brazilian Federative Units, corroborating other studies, which have indicated regions with the highest incidences.29–31 In other words, when comparing the different macroregions in terms of the number of deaths per 100,000 inhabitants (Fig. 2), there was a more significant decrease in deaths rates in the Southeast and South regions than in other regions (North, Northeast, and Midwest). This decrease in the deaths rates in the two regions mentioned above is certainly influenced by the presence of a better network of medical services as well as by the greater availability of healthcare facilities, enabling earlier diagnosis and more effective treatment.30
In addition, data from the Southeast and South regions of Brazil can be explained by better implementation of prevention and control measures, such as active search for cases and health education of the population. The data showed no significant correlation in the Northeast, North, and Midwest regions. There have been no significant reductions in death rates in these regions. Therefore, these data are positive since there has been a significant increase in the population in these regions in recent years. In the case of the Southeast and South regions, the data are very promising because, with a significant reduction in the number of deaths, it is possible to predict that in the coming years, there may be acceptable levels of death rates for this disease in the population.
Correlations were found between the rates of new cases of leprosy per 100,000 inhabitants and rates of disability, cure and mortality in different Brazilian states. A higher rate new cases of leprosy in two states of the Federation, the states of Mato Grosso and the state of Tocantins, located in the Midwest and north region, respectively (Fig. 3) throughout the study period. The distribution of cases is quite heterogeneous among the Brazilian macro-regions.32,33 In this study, there was a predominance of cases of Hansen's disease in the Midwest and North regions corroborating the data found in the two states, Mato Grosso and Tocantins. The higher rates of new cases in the states of Mato Grosso and Tocantins indicate the need to assess the associated factors, such as economic factors, health structure, and fewer preventive measures. In fact, in the regions where the two states are located, there are fewer control actions and other peculiarities that promote a higher incidence and mortality.34
Another important point analyzed in this study was the possible correlations between the number of new cases of Hansen's disease and the rates of physical disability and cure. The data showed a significant loss of physical capacity in patients with leprosy. These findings are related to the difficulty in making an early diagnosis. In other words, it appears that primary health policies are not sufficient to reach the entire population affected by Hansen's disease. The greater the degree of physical disability, the longer the patient has not received adequate treatment. Thus, a worrying scenario is observed, since the presence of physical disability leads to high treatment costs and the need to implement policies in order to improve the skills of health workers for early diagnosis and treatment of the disease. Consequently, avoiding sequelae and ensuring a better recovery for those who already have physical disability.28
Concerning cure rates among the Brazilian macro-regions, it was observed that the Southeast and South regions had higher rates among the new cases of leprosy in the evaluated period. Furthermore, the higher the cure rate, the lower the death rate per 100,000 inhabitants. These results suggest that these two regions have health services that meet the parameters established by the PEL in Brazil, which includes not only expanding the network of diagnosis and care for people affected by Hansen's disease but also an effective treatment network, thus providing greater success in curing the disease.34
The discrepancy in the period available and validated for notifications of new cases and death due to leprosy through the information system was a limitation of the study. In addition, there is underreporting of the disease in some regions of the country.35,36 which may limit the strengthen and consistency of our findings. The consolidation and dissemination of properly qualified health professionals trained in prevention, early detection of the disease and in its treatment can guarantee better results in the control of leprosy.
This work was supported by Fundação de Amparo à Pesquisa do Estado Minas Gerais (FAPEMIG), National Council for Scientific and Technological Development (CNPq), and Coordination for the Improvement of Higher Education Personnel (CAPES; Finance code 001).