To investigate the association of leprosy with hepatitis B virus (HBV) infection, as yet unknown for South Brazil, we assessed hepatitis B virus coinfection in 199 South Brazilian leprosy patients (119 lepromatous, 15 tuberculoid, 30 borderline, 12 undetermined and 23 unspecified) and in 681 matched blood donors by screening for the hepatitis B virus markers HBSAg and anti-HBc, using ELISA. Positive samples were retested and anti-HBc+ only samples were tested for the hepatitis B surface antibody (anti-HBs). There was a strong association between leprosy and hepatitis B virus infection (OR=9.8, 95% CI=6.4–14.7; p=0.004·E−30), as well as an association between HBV infection and lepromatous leprosy, compared to other forms (OR=2.4, 95% CI=1.2–4.8; p=0.017). We also found that confinement due to leprosy was associated with hepatitis B virus infection (OR=3.9, 95% CI=2.1–7.4; p=0.015·E−3). Leprosy patients are susceptible to develop hepatitis B virus infection, especially lepromatous. Institutionalized patients, who probably present a stronger Th2 response, have higher risk of being exposed to hepatitis B virus. This clearly emphasizes the need for special care to leprosy patients in preventing hepatitis B virus coinfection in South Brazil.
Leprosy is a long known infectious disease and still represents a major cause of morbidity and mortality in developing countries. According to data of the World Health Organization, more than 30,000 people are affected in the Americas.2 In 2012 alone, there were 406 new admissions to Brazilian hospitals due to leprosy. In South Brazil, 36,628 patients were diagnosed with the disease in 2011 and 10.9% presented grade 2 disability (11.4% in Paraná state), which is the highest rate in the country. South Brazil also has the 7th highest rate in Brazil of new case detection of leprosy with incapacity: 93.8%.1
Many leprosy patients also have positive markers for other infections, such as HIV and HBV (hepatitis B virus).3–7 In central Brazil, leprosy along with hepatitis B has been the subject of former investigations,6,7 but nothing is known about this disease association in other Brazilian regions. In this work, we aimed to describe leprosy–HBV epidemiology in South Brazil.
Materials and methodsSubjects and samplesTwo hundred and three patients with leprosy from three different treatment centers were selected. Twenty-three of them were attended at Universidade Federal do Paraná’s Clinical Hospital (HC-UFPR), 71 at Paraná’s Hospital of Sanitary Dermatology (PHSD) and 109 at Regional Center of Specialities-Barão (CRS-Barão). The patients from now on referred as “institutionalized” are 60 patients hospitalized permanently in PHSD. The high prevalence of institutionalized patients is explained by their social conditions. Since they presented advanced forms of leprosy or were abandoned by their families, confinement was the best way to assist them. Four patients had to be excluded from the study. Two of them did not show up to have their blood samples taken, and access to the medical records was not possible for another two. Thus, 199 patients were designated as “cases” for all analysis purposes.
The patients were also divided in groups according to the clinical classification proposed by Ridley and Jopling.8 Diagnosis at presentation was lepromatous leprosy for 119 patients (59.8%), tuberculoid leprosy for 15 (7.5%), and borderline leprosy for 30 patients (15.1%); 12 patients (6%) had an undetermined form of leprosy, and 23 (11.6%) were unspecified. Those with unspecified form of the disease were excluded from the analysis of clinical forms (176 cases were considered).
Six hundred and eighty-one blood donors tested for anti-HBc from HC-UFPR Hemotherapy Service were selected as controls. Cases (average age of 52±16 years) and controls (average age of 45±12 years) were also matched for age-range groups. The first group comprised nine patients and 36 controls between 15 and 24 years of age. The second group had 75 patients and 200 controls between 25 and 49 years, and the third one, 115 cases and 345 controls above 50 years old. 77 (38.7%) patients and 269 (39.5%) controls were female, whereas 122 (61.3%) patients and 412 (60.5%) controls were male.
Patients and controls were selected in 2002 and 2003. The study was approved by the ethics committee of the Clinic Hospital and Health State Department, and all subjects were asked to give written an informed consent for their participation and to answer a standardized questionnaire to determine the history of the patients regarding possible blood transfusion and the existence of other infectious diseases.
HBV testingSeven mL of blood were taken from each subject. The samples were centrifuged and serum aliquots stored at −20°C before HBV testing. An ELISA was used to detect the presence of anti-HBc (antibodies against HBV core antigen) (MONOLISA® a-HBc PLUS, BIO-RAD, Marnes La Coquette, France). To search for HBsAg, a sandwich ELISA was performed (MONOLISA® a-HBc PLUS, BIO-RAD, Marnes La Coquette, France). Patients positive for anti-HBc alone (HBsAg negative) were tested by microparticle enzyme immunoassay (MEIA) to detect antibodies against hepatitis B surface antigen (anti-HBs) (Murex anti-HBs, Murex Biotech Limited, Temple Hill, United Kingdom). All positive samples were retested using the same methods.
StatisticsFrequencies were compared using Fisher's exact test and odds ratios with the respective 95% confidence limits. Logistic regression models were used to adjust results for age, gender and assistance using the SPSS 13.0 software (IBM, USA).
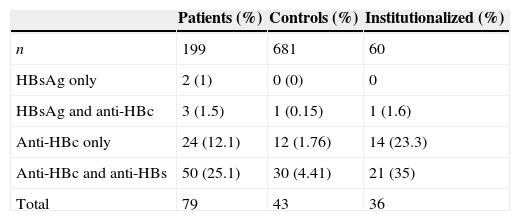
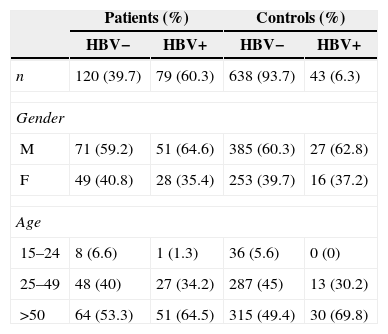
ResultsOf the leprosy patients, 39.7% were positive for HBV markers, in contrast to 6.3% of the control group (Table 1), suggesting a strong association between leprosy and HBV infection (79/199 vs. 43/681; OR=9.77, 95% CI=6.42–14.86; p<0.004·E−30). Of the leprosy patients, 26 (13.1%) received blood transfusion, of which twelve were positive for HbsAg and/or anti-HBc. Even after the exclusion of these possibly intravenously HBV-infected patients, leprosy and HBV infection remained associated (67/173 vs. 43/681; OR=9.38, 95% CI=6.08–14.48; p=0.006·E−24). There was no difference for HBV positivity according to gender and age (p=0.117, Table 2). The following co-infections were also observed in HBV+ leprosy patients: seven were positive for syphilis, six for HCV, two for Chagas disease, one for HCV and syphilis and one for syphilis and Chagas disease.
Different HBV infection markers in leprosy patients and controls.
| Patients (%) | Controls (%) | Institutionalized (%) | |
|---|---|---|---|
| n | 199 | 681 | 60 |
| HBsAg only | 2 (1) | 0 (0) | 0 |
| HBsAg and anti-HBc | 3 (1.5) | 1 (0.15) | 1 (1.6) |
| Anti-HBc only | 24 (12.1) | 12 (1.76) | 14 (23.3) |
| Anti-HBc and anti-HBs | 50 (25.1) | 30 (4.41) | 21 (35) |
| Total | 79 | 43 | 36 |
n, number of individuals.
Prevalence of HBV positivity according to gender and age.
| Patients (%) | Controls (%) | |||
|---|---|---|---|---|
| HBV− | HBV+ | HBV− | HBV+ | |
| n | 120 (39.7) | 79 (60.3) | 638 (93.7) | 43 (6.3) |
| Gender | ||||
| M | 71 (59.2) | 51 (64.6) | 385 (60.3) | 27 (62.8) |
| F | 49 (40.8) | 28 (35.4) | 253 (39.7) | 16 (37.2) |
| Age | ||||
| 15–24 | 8 (6.6) | 1 (1.3) | 36 (5.6) | 0 (0) |
| 25–49 | 48 (40) | 27 (34.2) | 287 (45) | 13 (30.2) |
| >50 | 64 (53.3) | 51 (64.5) | 315 (49.4) | 30 (69.8) |
n, number of individuals.
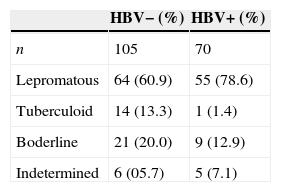
There was also an association between HBV infection and lepromatous leprosy, compared with the other leprosy forms (unspecified patients were excluded) (55/119 or 46.2% vs. 15/57 or 26.3%; OR=2.4, 95% CI=1.2–4.8; p=0.017·E−30, see Table 3). This remained significant in the logistic regression after correction for age and gender and was independent of confinement (p=0.018). We also found that confinement due to leprosy was associated with HBV infection (38/61 or 62.3% vs. 41/138 or 29.7%; OR=3.9, 95% CI=2.1–7.4; p=0.015·E−3). This result does also remain significant after correction for age and gender and was independent of the lepromatous status (p<0.0001). Two years after taking blood samples, the evaluation of confined HBV+ patients (38) showed that: 17 were released (9 women and 8 man; mean age=63), 12 were still confined (4 women and 8 men; mean age=46 years) and 8 passed away (1 woman and 7 men; mean age=73). Seven patients of HC-UFPR out clinic were HBV+, one of them was still there (an 87 year-old man), two had to be confined (one man and one woman; mean age=66) and three passed away (two women and one man; mean age=67). One of them presented a sudden and intense jaundice, dying a few days later.
Prevalence of HBV positivity according to different clinical forms of leprosy in patients from southern Brazil.
| HBV− (%) | HBV+ (%) | |
|---|---|---|
| n | 105 | 70 |
| Lepromatous | 64 (60.9) | 55 (78.6) |
| Tuberculoid | 14 (13.3) | 1 (1.4) |
| Boderline | 21 (20.0) | 9 (12.9) |
| Indetermined | 6 (05.7) | 5 (7.1) |
All the unspecified-clinical patients were excluded. n, number of individuals.
The positive association between leprosy and HBV infection has been repeatedly reported since the 1970s,6,9–20 although absence of association has also been found.21–27 Prevalence of HBV–leprosy co-infection depends on many factors, such as local endemicity and the sensitivity of methods used for HBV detection. Many authors considered only the prevalence of HBsAg.9,12,13,15,20,24,25,28,29 Others determined the prevalence of HBsAg and anti-HBs,14,17,19,22,30,31 whereas some screened only for anti-HBc.6,16,32 In contrast, we considered as HBV infected, patients having an active infection (HBsAg positive only, or anti-HBc and HBsAg positive) and patients who were exposed to the virus, with or without developing specific antibodies against it (positive for anti-HBc and/or anti-HBs). HBsAg or anti-HBc only results could be false positive, however, and patients with these results should thus be closely followed.33
In a recent study conducted in central Brazil, 25.5% of leprosy patients were positive for serological HBV markers and 2.6% had active disease.7 These figures were very similar to the results with a much larger leprosy population in Senegal (n=987).19 Between 1973 and 1977, the prevalence of HBV positivity in that setting was 25.5% and between 1982 and 1986, it was 23.0%. Thus HBV/leprosy co-infection is not exclusive to southern Brazil. Nevertheless, we encountered a much stronger association (60.3% of co-infection), possibly because of epidemiological differences and/or different sensitivity of HBV detection assays (radioimmunoassay vs. enzyme linked immunoassay).
As previously stated by others, there was no relevant association between co-infection and gender.10,19 This suggests that the susceptibility to leprosy/HBV co-infection is not directly linked to genes on the X chromosome or associated to sex-specific hormones. Also, analyzing age groups, we found that both leprosy patients and control groups show the same pattern of HBV infection. Age seems not to influence co-infection, as there was no difference in the distribution of co-infected cases according to age groups.
The higher prevalence of HBV positivity in patients with the lepromatous form of the disease was also expected.34,35 Lepromatous leprosy is characterized by a Th2-type immune response.36 This pattern of inflammatory response is also responsible for lowering the viral clearance of HBV.37 Thus, lepromatous leprosy patients are probably unable to perform a satisfactory clearance of HBV, becoming vulnerable to co-infection.
We also confirmed the higher prevalence of HBV infection in patients kept in “leprosariums”.6,13,22 Since hepatitis B is transmitted person-to-person via body fluids,21 patients who are confined have higher risk to be infected, because HBV positive and HBV negative subjects share the same space. It is interesting that Rosa et al. also found that institutionalized patients in central Brazil had almost fourfold risk of being HBV infected, if compared to outpatients.6
Thus, leprosy/HBV co-infection is not uncommon in South Brazil. Leprosy patients are susceptible to develop HBV infection, especially lepromatous and institutionalized patients, who probably present a higher risk of being exposed to the HBV. This clearly emphasizes the need for special care to leprosy patients in preventing HBV co-infection.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the subjects of this investigation for their consent to participate and Lablife Ltd. for providing all the ELISA kits, as well as the Serology Lab of Hemotherapy Service from Clinic Hospital of Federal University for their technical support, Dr. Ewalda Von Rosen Seeling Stahlke and staff from the Health State Department of Paraná and Sanitary Dermatology Hospital of Paraná for their collaboration in the selection of the patients. A.B.W. Boldt and I.J.T. Messias-Reason grants were funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior). The funding sources had no involvement in study design, collection, analysis and interpretation of data, writing of the report and in the decision to submit the manuscript for publication.