Lemierre syndrome is characterized by acute septic thrombophlebitis of the internal jugular vein (IJV) that develops after an oropharyngeal infection, and can be complicated by septic emboli to lungs and other organs. The most frequent causative agent is Fusobacterium necrophorum, an anaerobic bacillus found in normal oropharyngeal flora. Staphylococcus aureus has emerged as a cause of Lemierre syndrome in the last decade. We report a case of a 24-year-old man who developed septic IJV thrombosis and necrotizing pneumonia due to S. aureus from an infected hematoma in the right sternocleidomastoid muscle. Antibiotics are the mainstay of therapy with few cases needing anticoagulation. A good outcome is dependent upon an awareness of the condition, a high index of suspicion, and prompt initiation of antibiotic therapy. Recognition of S. aureus as a cause of Lemierre syndrome can guide the choice of initial antibiotics to cover this virulent pathogen.
Lemierre syndrome or postanginal sepsis (necrobacillosis) is characterized by septic thrombophlebitis of the internal jugular vein (IJV) with frequent metastatic infections, usually due to anaerobic organisms.1 We report a case of Lemierre syndrome complicating an infected neck hematoma due to methicillin-resistant Staphylococcus aureus (MRSA), which was associated with bilateral septic pulmonary emboli and empyema. The patient was successfully treated with antibiotics, anticoagulation, surgical evacuation of the neck abscess and chest tube drainage of the empyema. Recognition of S. aureus as a cause of postanginal sepsis can guide the choice of initial antibiotics to cover this virulent pathogen.
Case presentationA 24-year-old man presented with a 3-day history of painful swelling of the right neck and shoulder area. Initially, he attributed his symptoms to a “pulled muscle” as he did flooring work and regularly carried carpets as part of his work. However, the onset of fever, nausea and lethargy prompted him to seek medical attention. His medical history was unremarkable except for a dental infection requiring a right mandibular tooth extraction three weeks ago. On admission, he had a temperature of 100°F with blood pressure of 102/70mmHg and a pulse of 98 beats per minute. He had erythema and tender induration of the right anterior neck, extending towards the right shoulder. Oral exam did not reveal any tooth decay or pharyngeal exudates. He had scattered crackles over bilateral lung fields. Heart and abdominal exam was unremarkable.
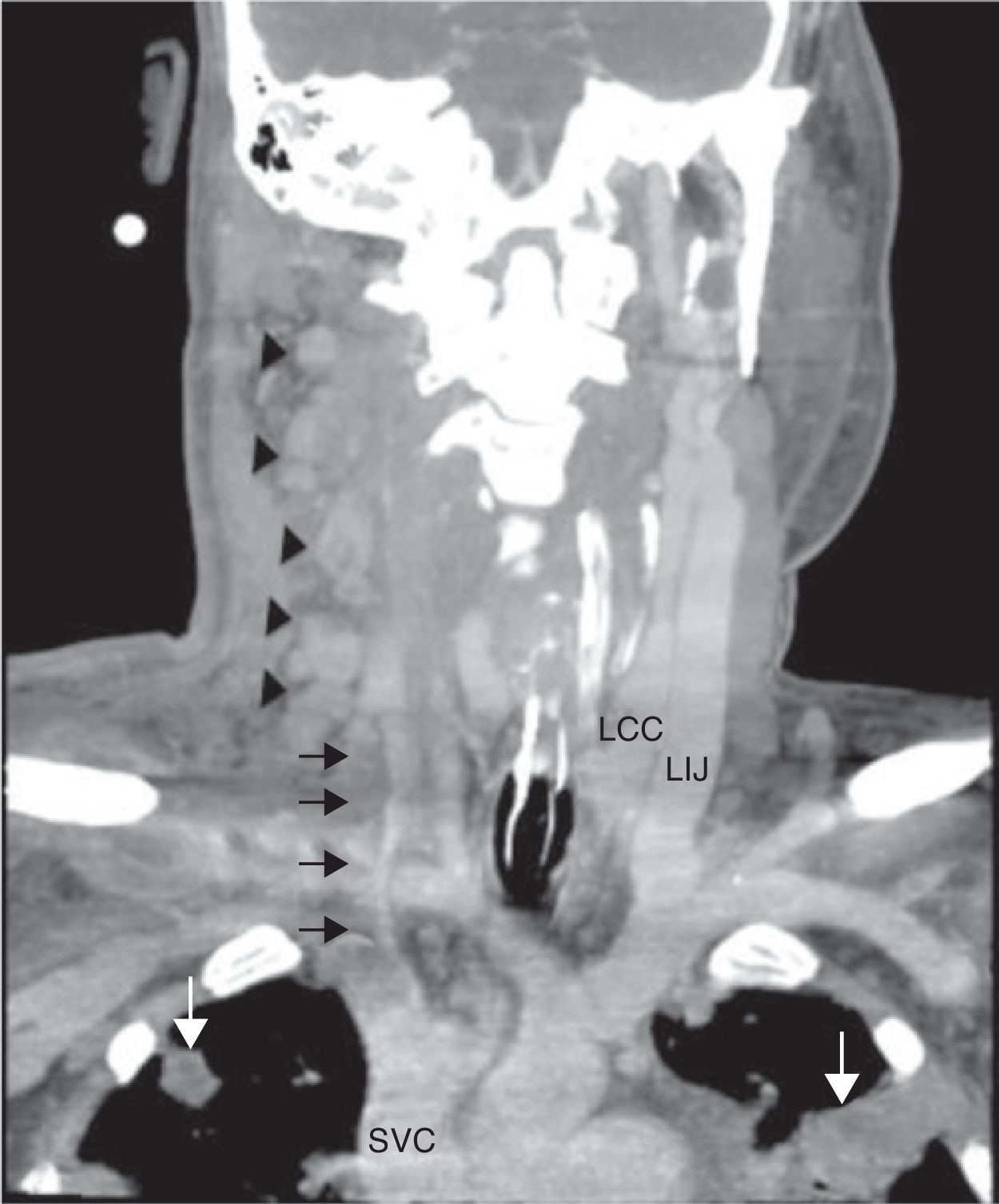
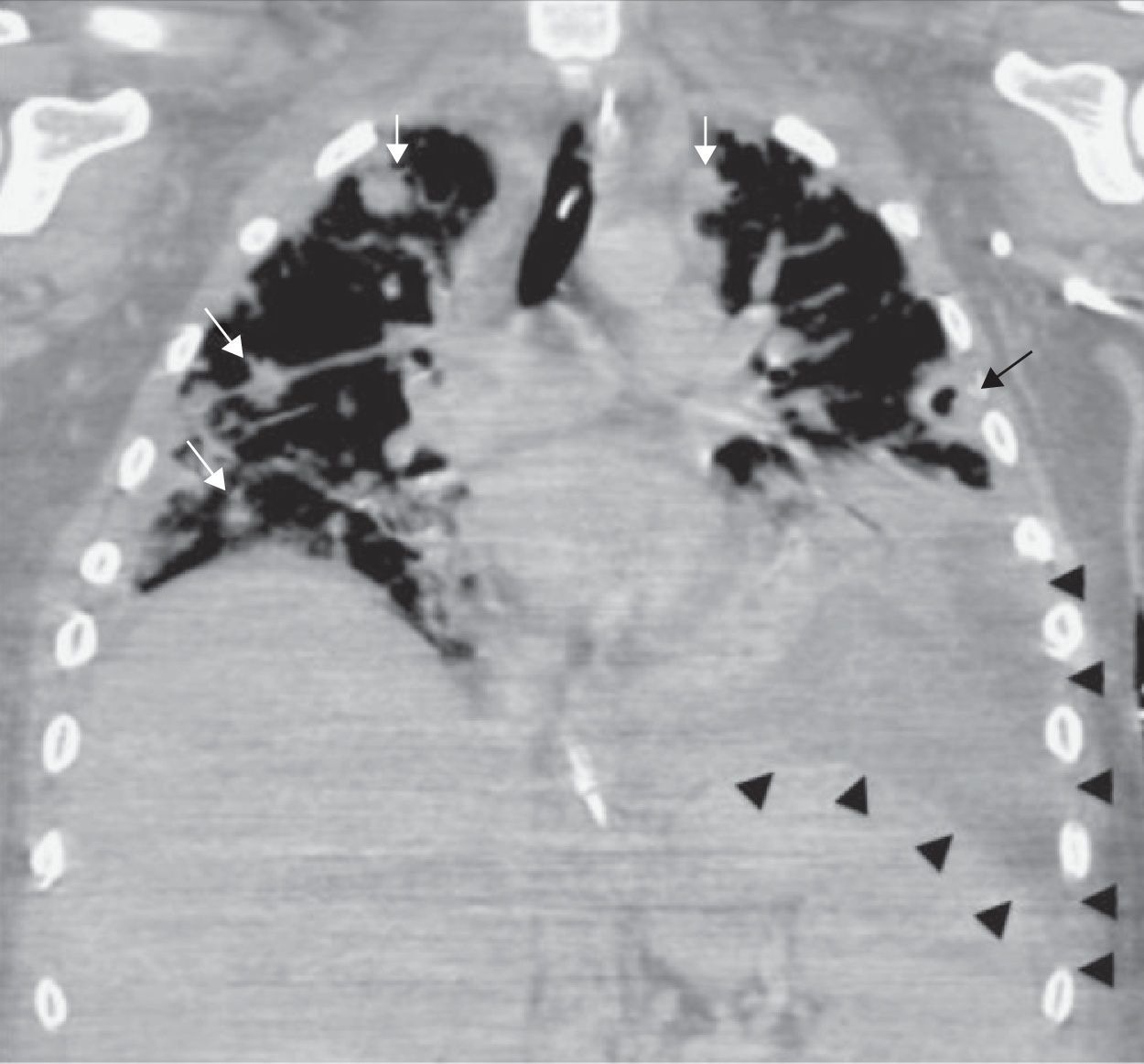
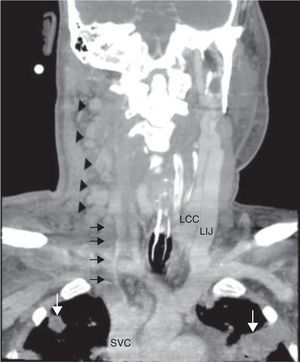
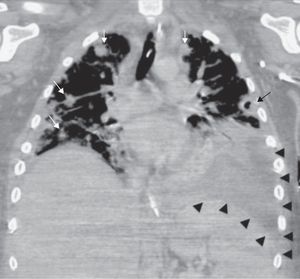
Laboratory findings included an elevated leukocyte count of 20,000/μL with 82% neutrophils. Serum creatinine and liver function tests were within normal range. A chest X-ray showed nodular alveolar opacities. A computed tomography (CT) of the neck revealed an infected hematoma in his neck along with a thrombus in the right internal jugular vein extending to subclavian confluence along with extensive cervical lymphadenopathy (Fig. 1). He was admitted for intravenous antibiotics (vancomycin, and piperacillin-tazobactam) and anticoagulation with heparin. He developed progressive dyspnea over the next two days and required intubation and mechanical ventilation for hypoxic respiratory failure. A chest CT revealed multiple peripherally enhancing nodular areas with central cavitation and surrounding consolidation suggestive of septic emboli, and bilateral parapneumonic pleural effusions (Fig. 2). A diagnosis of Lemierre syndrome was made and clindamycin was added to his regimen. Surgical exploration of the neck revealed a large infected hematoma in and around the sternocleidomastoid muscle, which was debrided and a drain was left in place (Fig. 3). Diagnostic thoracentesis revealed exudative pleural fluid with a pH of 7.26 consistent with empyema for which bilateral chest tubes were placed.
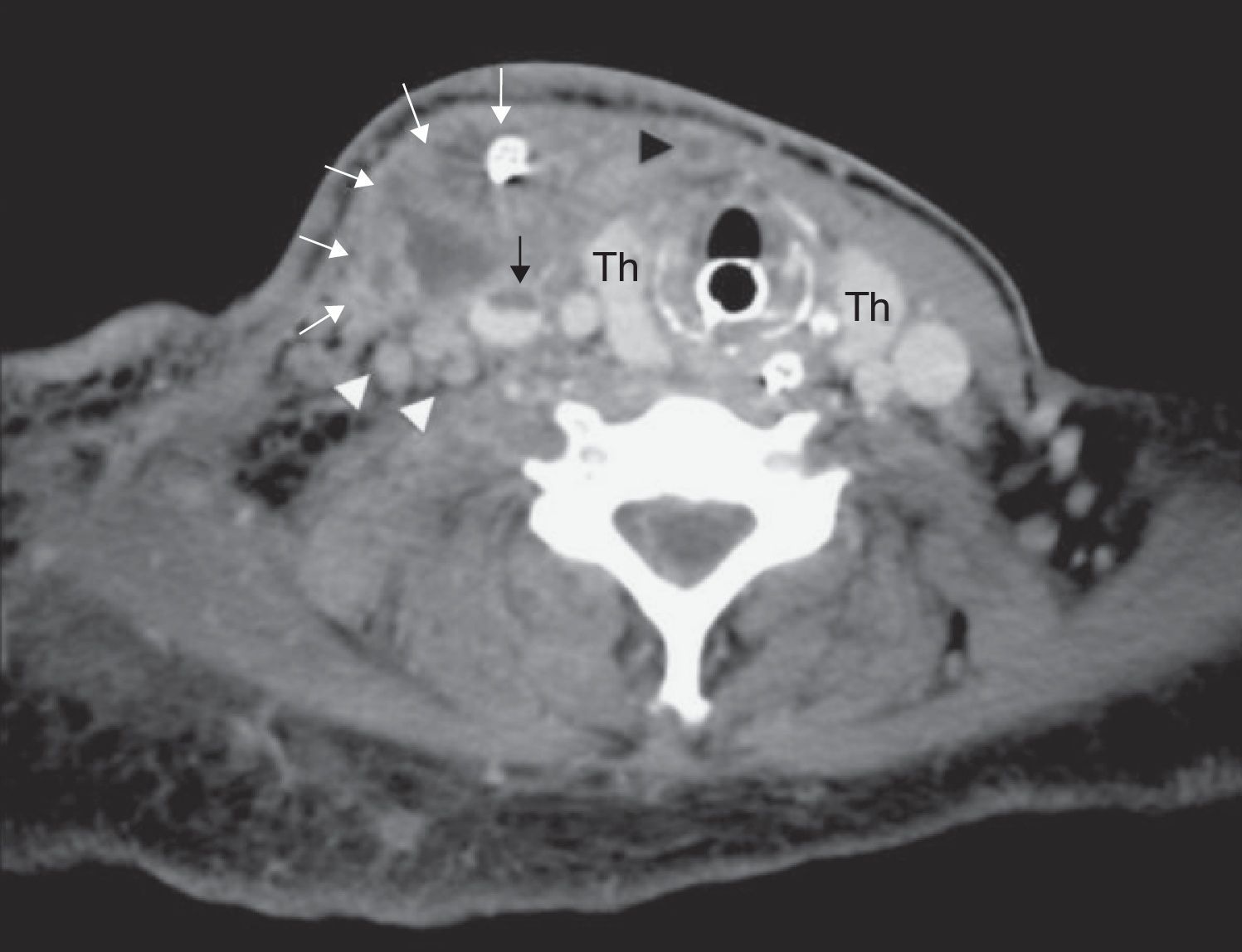
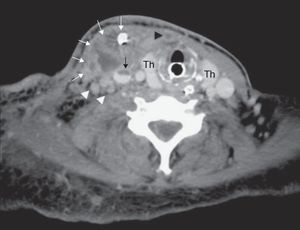
Post-operative CT at the level of the thyroid gland (Th) shows a complex abscess in the right sternocleidomastoid muscle with drain in situ (white arrows). Notice the partially thrombosed right jugular vein (black arrow), completely thrombosed anterior jugular vein (black arrowhead) and enlarged right internal jugular lymph nodes (white arrowheads).
Blood, wound and sputum cultures grew MRSA. All antibiotics other than vancomycin were stopped. The trough vancomycin levels were noted to be low, so the dose was increased from 1500mg every 12h to 1500mg every 8h with trough levels of 18–20μg/mL. Patient was weaned off ventilator support after two weeks, followed by removal of the neck and chest tubes. Repeat blood cultures remained sterile. He was bridged from heparin to a 3-month course of warfarin, and was discharged on vancomycin to complete a total of 6 weeks of antibiotics.
DiscussionFirst described in 1936, Lemierre syndrome is an uncommon but potentially life-threatening condition characterized by upper respiratory tract infection, thrombophlebitis of the neck veins, anaerobic sepsis, and systemic dissemination of septic emboli.1 It is usually secondary to oropharyngeal infection; other causes include dental, sinus and ear infections, intravenous drug abuse and catheterization of the IJV.2 Thrombosis of the IJV is usually caused by extension of infection to the lateral pharyngeal wall or parapharyngeal space. The most common pathogen is Fusobacterium necrophorum, a gram-negative anaerobe responsible for approximately 70% of cases.3 The most frequently involved sites of septic metastases are the lungs, followed by the joints.4 Lung findings may include infiltrates, nodular densities, cavitations, and pleural effusion.
S. aureus is a gram-positive coccal bacterium that is part of the normal flora on skin and nasal passages. It is an important cause of skin and soft tissue infections, and septic abscesses. However, it has rarely been reported in the context of postanginal sepsis. Excluding cases related to central venous catheters, 12 cases of septic internal jugular thrombophlebitis related to S. aureus have been reported in the literature between 1965 and 2011, and all of them have been reported after 2001.5 There was one fatality in these 12 cases. Only three of the 12 cases were due to a methicillin-sensitive strain of S. aureus. All cases were complicated by pulmonary emboli. In our patient, the source of MRSA was felt to be related to dental infection, which then seeded a preexisting traumatic neck hematoma (patient carried heavy carpets on his shoulder). Community-associated MRSA (CA-MRSA) has been recognized for almost two decades. While initially CA-MRSA infections were more common in certain groups such as homeless, injecting drug users, prisoners, sports participants and military, these strains now represent a major part of staphylococcal infections in the community.6 Also, the CA-MRSA strains are different from healthcare-associated MRSA strains and can be distinguished by certain molecular techniques.6 Given the increased prevalence and admission to hospitals of individuals with CA-MRSA, it is postulated that in the USA, CA-MRSA will become the dominant MRSA strain in healthcare facilities, and cause increased severity of infections and longer hospital stays.7
Broad spectrum antibiotics, including anaerobic coverage should be instituted promptly on suspicion of postanginal sepsis. Antibiotics should then be tailored depending on culture and sensitivity results. Clindamycin is suitable monotherapy for Fusobacterium infections. For MRSA infections, treatment options include vancomycin, daptomycin, linezolid, and telavancin.5 There is no standard duration of therapy, but in general antibiotics should be continued until clot resolution, which usually occurs after four to six weeks of therapy.5 Daptomycin should not be used for cases with lung involvement as it is inhibited by surfactant. Role of anticoagulation is controversial. It should be considered for extension of thrombus to the cavernous sinus and continuing septic emboli despite antibiotics.8 Surgical ligation of the internal jugular veins, though commonly performed in the preantibiotic era, is rarely used now, mainly for patients with recurrent episodes of embolization despite treatment with antibiotics.9 Lemierre syndrome is associated with increased morbidity and prolonged hospital stays. On a recent review of 114 cases of Lemierre syndrome, the pooled mortality was 5%.10 There appears to be an increase in Lemierre syndrome cases, perhaps due to antibiotic resistance or changes in antibiotic prescription patterns.8
ConclusionIn conclusion, a high index of suspicion is important for timely diagnosis and prompt initiation of appropriate antibiotics, including anaerobic coverage, is essential to decrease the morbidity and mortality associated with Lemierre syndrome. It is important to recognize S. aureus as an emergent cause of Lemierre syndrome in the last decade and initial antibiotic therapy should cover for this possibility.
Conflict of interestThe authors declare no conflict of interest.