Brazilian borreliosis (BB) disease is an infectious disease transmitted by ticks that mimics Lyme disease (LD) from the Northern Hemisphere. The BB clinical picture is characterized by a pathognomonic skin lesion (migratory erythema) and joint, neurological, cardiac and psychiatric symptoms. Innate and Th1/Th17 adaptive immunity seem to play an important role in the pathogenesis of Lyme disease.
ObjectiveThe aim of this study was to characterize the role of innate and Th1/Th17 adaptive immunity in BB patients with acute (<3 months) and convalescent (>3 months) disease.
MethodsFifty BB patients (28 with acute and 22 with convalescent disease) without treatment and 30 healthy subjects were evaluated. Levels of 20 cytokines or chemokines associated with innate and Th1/Th17 adaptive immunity were analyzed using Luminex (Millipore Corp., Billerica, MA).
ResultsOverall, BB patients had increased levels of IL-8 (6.29 vs 2.12 p = 0.002) and MIP-1α/CCL3 (5.20 vs 2.06, p = 0.030), associated with innate immunity, and MIP3B/CCL19 (Th1; 297.86 vs 212.41, p = 0.031) and IL-17A (Th17; 3.11 vs 2.20, p = 0.037), associated with adaptive immunity, compared with the levels of healthy controls. When comparing acute BB vs. convalescent BB subjects vs. healthy controls, IL-1β, IL-8 and MIP-1α/CCL3 (innate mediators) levels were highest in patients in the acute phase of disease (p < 0.05). TNF-α was associated with disseminated symptoms and with humoral reactivity against Borrelia burgdorferi. IL-10 was significantly correlated with IL-6 (r = 0.59, p = 0.003), IL-8 (r = 0.51, p < 0.001), MIP-1α/CCL3 (r = 0.42, p < 0.001) and MIP-3β/CCL19 (r = 0.40, p = 0.002) in all BB patients.
ConclusionsThis is the first study describing that innate and Th1/Th17 adaptive immunity play a crucial role in BB disease. Furthermore, innate mediators are particularly important in acute BB disease, and TNF-α is associated with evolution of BB symptoms.
Brazilian borreliosis (BB) was first described in 1992 by Yoshinari et al. in Cotia, São Paulo state, Brazil.1 BB is similar to Lyme disease (LD), the major tick-borne zoonosis from Northern Hemisphere. LD, caused by Borrelia sp. from the B. burgdorferi sensu lato complex, is transmitted by ticks classified into the Ixodes ricinus group, its clinical manifestations are the pathognomonic skin lesion (erythema migrans) on the site of the tick bite and articular, neurologic and cardiac symptoms.2 BB, also known as Baggio-Yoshinari Syndrome, is defined as a zoonosis caused by bacterial infection transmitted by tick bite, which have skin, neurological, articular and cardiac manifestations.3,4 BB patients have clinical manifestations similar to those of LD patients, although they can show recurrent symptoms after treatment.4 The etiologic agent of BB disease is a spirochete classified into the Borrelia burgdorferi sensu lato complex, but this bacteria have atypical morphology and are uncultivable in specific culture medium.5 The BB vectors are native species of the country.6,7 In the BB acute phase, defined as disease with less than three months of evolution after the tick bite, almost half of patients develop erythema migrans (EM) associated with flu-like symptoms.4 The early disseminated stage of BB disease may be characterized by multiple new lesions, meningitis, facial palsy, arthritis, a skin lesion called borrelial lymphocytoma, fever, weakness, headache, muscular pain, cough, arthralgia and sensitive radicular neuropathy.8,9,10 Without antibiotic treatment after initial symptoms, neurologic, articular, cardiac and skin manifestations are observed. Studies on immune responses have shown that inflammatory mediators are correlated with manifestation of LD symptoms. Elevated levels of T helper 1 cell (Th1) cytokines and chemokines have been reported in the cerebrospinal fluid and serum of LD patients in active phase, neuroborreliosis and post-treatment Lyme disease syndrome.11,12,13,14 In addition, the role of adaptive T helper 17 (Th17) cytokines was associated with the pathogenesis of Lyme arthritis.15,16 Strle et al. showed higher levels of cytokines and chemokines from innate immunity and TH1 cells in serum samples and in the supernatant of macrophage cultures stimulated by Borrelia sp. that were recovered from EM skin lesions of acute LD patients.17 The innate and Th1 and TH17 adaptive immunity were described in all phases of LD.
The present study aimed to analyze the cytokines and chemokines of innate and Th1 and TH17 adaptive immune inflammatory responses in the serum of acute and convalescent BB disease for the first time.
Materials and methodsPatients and samplesWe have conducted a prospective study of 50 Brazilian borreliosis (BB) patients who met the criteria for the diagnosis of disease proposed by Yoshinari et al.4
All of them had a history of EM that was manifested before or at the time of anamnesis. All patients had a history of tick bit and none had been treated with antibiotics. The levels of inflammatory mediators were determined in serum samples sent from medical care centers. Therefore, blood was collected at the same time of medical appointment, when the physician filled out and signed a standardized questionnaire with demographic data, history of tick bites, symptoms onset after tick bite, and clinical presentation. The patients did not have periodic study visits. The clinical symptoms of BB patients were characterized regarding the presence of dermatologic, articular, neurologic and cardiac symptoms at the time of medical appointment and blood collection. Patients with symptoms with less than three months of disease after tick biting were defined as acute BB disease and those with more than three months as convalescent illness. Patients’ serum samples and respective standardized questionnaires were sent to the Brazilian Central Laboratories of Public Health of many states, since BB cases occured in every region: four northern, two northeastern, nine eastern-central, 10 southern, and 25 southeastern. All samples were stored at −80 °C. The control group consisted of 30 healthy administrative workers with contact with pathogens in the laboratory, where this study was developed, to minimize false reactivity. All patients and healthy donors provided written informed consent, and the study was approved by the Human Research Committee of Hospital das Clinicas, Faculdade de Medicina, Universidade de São Paulo (CAPPesq).
Brazilian borreliosis serum diagnosisAntibody responses (immunoglobulin G [IgG] and immunoglobulin M [IgM]) to B. burgdorferi American G39/40 strain whole-cell sonicate antigen were determined by ELISA (enzyme-linked immunoassay) and Western blotting (WBlot) with in-house tests according to previously published papers.1
Innate and Th1/Th17 adaptive immunity cytokine and chemokine analysesThe levels of 20 cytokines and chemokines associated with innate immunity: MIP-1α (macrophage inflammatory protein-1 alpha) or CCL3 (chemokine C-C motif-ligand 3), MIP-1β or CCL4, IL (interleukin)-1β, IL-6, IL-8, IL-10, TNF (tumor necrosis factor)-α and IFN (interferon)-α, adaptive Th1 (T helper cell – 1) immunity: IFN-µ, CXCL9 (chemokine C-X-C motif-ligand 9) or MIG (monokine — induced by gamma interferon), IP-10 (interferon gamma-induced protein 10) or CXCL10, IL-12p40, IL-12p70 and MIP-3β or CCL19, and adaptive Th17 immunity: IL-17A, IL-E, IL-17F, IL-21, IL-20, and IL-23 were assessed in serum samples from acute and convalescent BB patients and healthy subjects. The four panels used were: 1. HCYP2MAG-62K-01; 2. K2-HTH17MAG-14K-05; 3. HCYTOMAG-60K-12; and 4. HCYP3MAG-63K-02 (Multiplex Multiple Analyte Profiling kits for Human Cytokine/Chemokine Magnetic Bead Panel, Millipore Corp., Billerica, MA). Analyses were quantified using a Magpix test instrument (Luminex Corp., Austin, TX) and xPONENT 4.2 software (Luminex). Concentrations of cytokines (pg/mL) were determined on the basis of the fit of a standard curve for mean fluorescence intensity versus concentration.
Statistical analysisDifferences in cytokine and chemokine levels between the total, acute or convalescent BB disease and healthy controls were assessed using Wilcoxon and Kruskal–Wallis nonparametric tests. The Pearson correlation test was used to analyze the associations between inflammatory mediators and some symptoms, inflammatory mediators and serologic reactivity, and cytokines and chemokines. A p-value ≤ 0.05 was considered statistically significant.
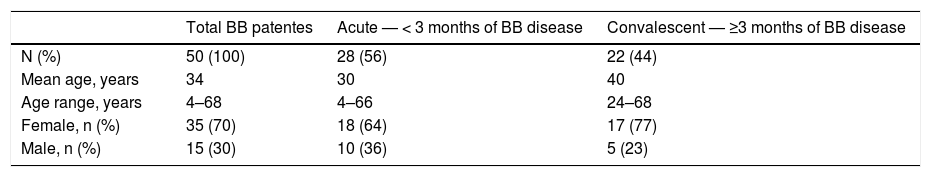
ResultsDemographic data of Brazilian borreliosis patientsThe mean age of the 50 BB patients was 34 (from 4 to 68) years. Thirty-five (70%) were female; 28 (56%) patients were in acute phase of disease (<3 months), mean age of 30 (from 4 to 66) years and 18 (64%) were female. Twenty-two (44%) patients were in the convalescent phase of illness, mean age of 40 (from 24 to 68) years and 17 (77%) were female (Table 1).
Demographic data of acute (<3 months) and convalescent (>3 months) Brazilian borreliosis (BB) patients.
| Total BB patentes | Acute — < 3 months of BB disease | Convalescent — ≥3 months of BB disease | |
|---|---|---|---|
| N (%) | 50 (100) | 28 (56) | 22 (44) |
| Mean age, years | 34 | 30 | 40 |
| Age range, years | 4–68 | 4–66 | 24–68 |
| Female, n (%) | 35 (70) | 18 (64) | 17 (77) |
| Male, n (%) | 15 (30) | 10 (36) | 5 (23) |
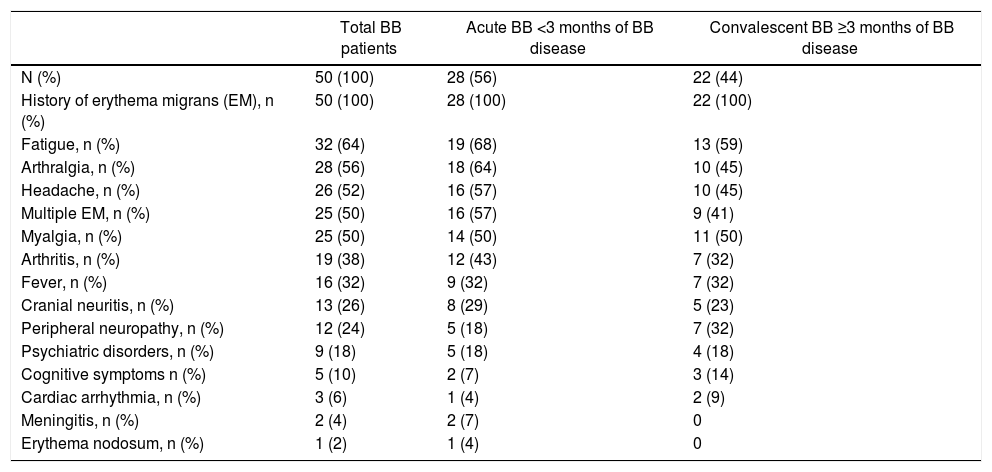
According to the inclusion criteria, all BB patients showed EM dermatologic lesions.4 The clinical manifestations of patient in all phases of disease are shown in Table 2. In addition, 24 BB patients had one to five symptoms, 20 had six to 10 symptoms and five had 11–14 clinical manifestations.
Clinical symptoms of Brazilian borreliosis (BB) patients.
| Total BB patients | Acute BB <3 months of BB disease | Convalescent BB ≥3 months of BB disease | |
|---|---|---|---|
| N (%) | 50 (100) | 28 (56) | 22 (44) |
| History of erythema migrans (EM), n (%) | 50 (100) | 28 (100) | 22 (100) |
| Fatigue, n (%) | 32 (64) | 19 (68) | 13 (59) |
| Arthralgia, n (%) | 28 (56) | 18 (64) | 10 (45) |
| Headache, n (%) | 26 (52) | 16 (57) | 10 (45) |
| Multiple EM, n (%) | 25 (50) | 16 (57) | 9 (41) |
| Myalgia, n (%) | 25 (50) | 14 (50) | 11 (50) |
| Arthritis, n (%) | 19 (38) | 12 (43) | 7 (32) |
| Fever, n (%) | 16 (32) | 9 (32) | 7 (32) |
| Cranial neuritis, n (%) | 13 (26) | 8 (29) | 5 (23) |
| Peripheral neuropathy, n (%) | 12 (24) | 5 (18) | 7 (32) |
| Psychiatric disorders, n (%) | 9 (18) | 5 (18) | 4 (18) |
| Cognitive symptoms n (%) | 5 (10) | 2 (7) | 3 (14) |
| Cardiac arrhythmia, n (%) | 3 (6) | 1 (4) | 2 (9) |
| Meningitis, n (%) | 2 (4) | 2 (7) | 0 |
| Erythema nodosum, n (%) | 1 (2) | 1 (4) | 0 |
Forty-eight percent (n = 24) of BB patients had positive serology (ELISA or WBlot). Borrelia burgdorferi G39/40 strain whole-cell sonicate antigen was used for detecting IgM or IgG antibodies. Until three months of illness, 50% (14/28) showed positive serology, and 45% (10/22) of patients with more than three months of clinical evolution had reactive antibodies to borrelial antigens.
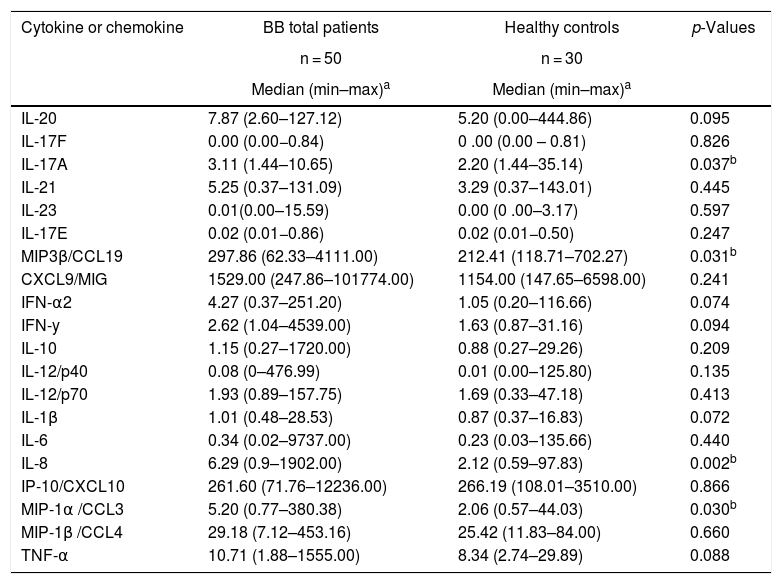
Innate immunity in acute and convalescent BB diseaseAnalysis of innate inflammatory mediators in sera from 50 BB patients showed that the levels of IL-8 (p = 0,002) and MIP-1α/CCL3 (p = 0.030) were significantly higher than the levels of healthy controls (Table 3). Three innate inflammatory mediators, IL-1β (p = 0.051), IL-8 (p < 0.001) and MIP-1α/CCL3 (p = 0.017) were significantly higher in acute phase patients of BB disease (<3 months) than in healthy controls (Table 4). In contrast, serum levels of innate inflammatory cytokines and chemokines from convalescent (≥3 months) BB patients were not significantly different than levels of healthy controls (Table 4). In BB patients, the innate proinflammatory cytokine TNF-α was correlated with higher number of clinical symptoms or disseminated infection (r = 0.28, p = 0.049). In addition, we evaluated the association between seropositivity against B. burgdorferi antigens and median levels of inflammatory mediators. The innate inflammatory cytokine TNF-α was associated with the humoral immunity (median 4.0678: 0.1400–9.4800, p = 0.042).
Comparison of innate and Th1/Th17 adaptive inflammatory mediators between total Brazilian borreliosis (BB) patients and healthy controls.
| Cytokine or chemokine | BB total patients | Healthy controls | p-Values |
|---|---|---|---|
| n = 50 | n = 30 | ||
| Median (min–max)a | Median (min–max)a | ||
| IL-20 | 7.87 (2.60–127.12) | 5.20 (0.00–444.86) | 0.095 |
| IL-17F | 0.00 (0.00−0.84) | 0 .00 (0.00 – 0.81) | 0.826 |
| IL-17A | 3.11 (1.44–10.65) | 2.20 (1.44–35.14) | 0.037b |
| IL-21 | 5.25 (0.37–131.09) | 3.29 (0.37–143.01) | 0.445 |
| IL-23 | 0.01(0.00–15.59) | 0.00 (0 .00–3.17) | 0.597 |
| IL-17E | 0.02 (0.01−0.86) | 0.02 (0.01−0.50) | 0.247 |
| MIP3β/CCL19 | 297.86 (62.33–4111.00) | 212.41 (118.71–702.27) | 0.031b |
| CXCL9/MIG | 1529.00 (247.86–101774.00) | 1154.00 (147.65–6598.00) | 0.241 |
| IFN-α2 | 4.27 (0.37–251.20) | 1.05 (0.20–116.66) | 0.074 |
| IFN-y | 2.62 (1.04–4539.00) | 1.63 (0.87–31.16) | 0.094 |
| IL-10 | 1.15 (0.27–1720.00) | 0.88 (0.27–29.26) | 0.209 |
| IL-12/p40 | 0.08 (0–476.99) | 0.01 (0.00–125.80) | 0.135 |
| IL-12/p70 | 1.93 (0.89–157.75) | 1.69 (0.33–47.18) | 0.413 |
| IL-1β | 1.01 (0.48–28.53) | 0.87 (0.37–16.83) | 0.072 |
| IL-6 | 0.34 (0.02–9737.00) | 0.23 (0.03–135.66) | 0.440 |
| IL-8 | 6.29 (0.9–1902.00) | 2.12 (0.59–97.83) | 0.002b |
| IP-10/CXCL10 | 261.60 (71.76–12236.00) | 266.19 (108.01–3510.00) | 0.866 |
| MIP-1α /CCL3 | 5.20 (0.77–380.38) | 2.06 (0.57–44.03) | 0.030b |
| MIP-1β /CCL4 | 29.18 (7.12–453.16) | 25.42 (11.83–84.00) | 0.660 |
| TNF-α | 10.71 (1.88–1555.00) | 8.34 (2.74–29.89) | 0.088 |
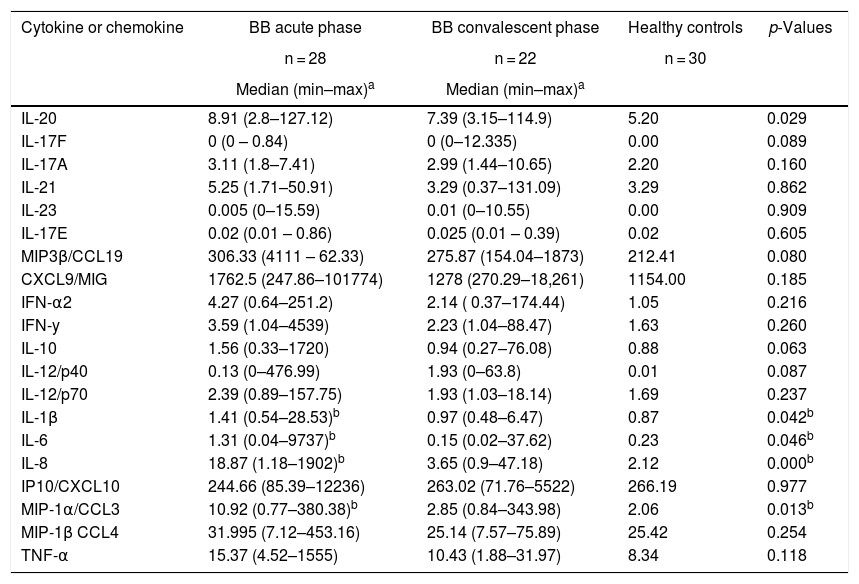
Comparison of innate and Th1/Th17 adaptive inflammatory mediators between acute (<3 months) or convalescent (≥3 months) Brazilian borreliosis (BB) patients and healthy controls.
| Cytokine or chemokine | BB acute phase | BB convalescent phase | Healthy controls | p-Values |
|---|---|---|---|---|
| n = 28 | n = 22 | n = 30 | ||
| Median (min–max)a | Median (min–max)a | |||
| IL-20 | 8.91 (2.8–127.12) | 7.39 (3.15–114.9) | 5.20 | 0.029 |
| IL-17F | 0 (0 – 0.84) | 0 (0–12.335) | 0.00 | 0.089 |
| IL-17A | 3.11 (1.8–7.41) | 2.99 (1.44–10.65) | 2.20 | 0.160 |
| IL-21 | 5.25 (1.71–50.91) | 3.29 (0.37–131.09) | 3.29 | 0.862 |
| IL-23 | 0.005 (0–15.59) | 0.01 (0–10.55) | 0.00 | 0.909 |
| IL-17E | 0.02 (0.01 – 0.86) | 0.025 (0.01 – 0.39) | 0.02 | 0.605 |
| MIP3β/CCL19 | 306.33 (4111 – 62.33) | 275.87 (154.04–1873) | 212.41 | 0.080 |
| CXCL9/MIG | 1762.5 (247.86–101774) | 1278 (270.29–18,261) | 1154.00 | 0.185 |
| IFN-α2 | 4.27 (0.64–251.2) | 2.14 ( 0.37–174.44) | 1.05 | 0.216 |
| IFN-y | 3.59 (1.04–4539) | 2.23 (1.04–88.47) | 1.63 | 0.260 |
| IL-10 | 1.56 (0.33–1720) | 0.94 (0.27–76.08) | 0.88 | 0.063 |
| IL-12/p40 | 0.13 (0–476.99) | 1.93 (0–63.8) | 0.01 | 0.087 |
| IL-12/p70 | 2.39 (0.89–157.75) | 1.93 (1.03–18.14) | 1.69 | 0.237 |
| IL-1β | 1.41 (0.54–28.53)b | 0.97 (0.48–6.47) | 0.87 | 0.042b |
| IL-6 | 1.31 (0.04–9737)b | 0.15 (0.02–37.62) | 0.23 | 0.046b |
| IL-8 | 18.87 (1.18–1902)b | 3.65 (0.9–47.18) | 2.12 | 0.000b |
| IP10/CXCL10 | 244.66 (85.39–12236) | 263.02 (71.76–5522) | 266.19 | 0.977 |
| MIP-1α/CCL3 | 10.92 (0.77–380.38)b | 2.85 (0.84–343.98) | 2.06 | 0.013b |
| MIP-1β CCL4 | 31.995 (7.12–453.16) | 25.14 (7.57–75.89) | 25.42 | 0.254 |
| TNF-α | 15.37 (4.52–1555) | 10.43 (1.88–31.97) | 8.34 | 0.118 |
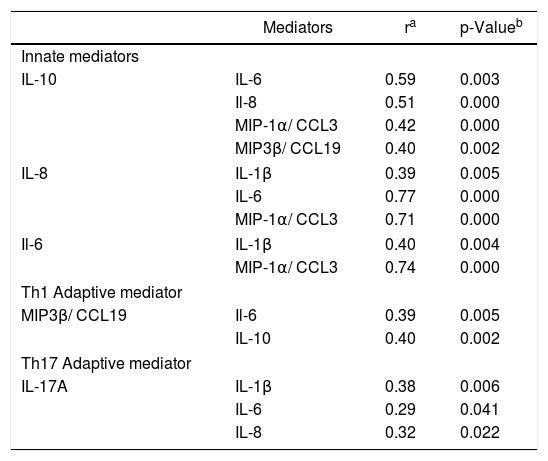
Furthermore, the median of IL-10 innate cytokine was correlated with IL-6 (r = 0.59, p = 0.003), IL-8 (r = 0.51, p = 0.000) and MIP-1α/CCL3 (r = 0.42, p = 0.000) innate mediators, as well as MIP-3β/CCL19 Th1 chemokine (r = 0.40, p = 0.002). In addition, the innate chemokine IL-8 was correlated with IL-1β (r = 0.39, p = 0.005), IL-6 (r = 0.77, p = 0.000) and MIP-1α/CCL3 (r = 0.71, p = 0.000). The correlation also was present between the innate cytokine IL-6 with IL-1β (r = 0.40, p = 0.004) and with MIP-1α/CCL3 (r = 0.74, p = 0.000) (Table 5).
Correlation between innate and Th1 /Th17 adaptive mediators in total BB serum samples.
| Mediators | ra | p-Valueb | |
|---|---|---|---|
| Innate mediators | |||
| IL-10 | IL-6 | 0.59 | 0.003 |
| Il-8 | 0.51 | 0.000 | |
| MIP-1α/ CCL3 | 0.42 | 0.000 | |
| MIP3β/ CCL19 | 0.40 | 0.002 | |
| IL-8 | IL-1β | 0.39 | 0.005 |
| IL-6 | 0.77 | 0.000 | |
| MIP-1α/ CCL3 | 0.71 | 0.000 | |
| Il-6 | IL-1β | 0.40 | 0.004 |
| MIP-1α/ CCL3 | 0.74 | 0.000 | |
| Th1 Adaptive mediator | |||
| MIP3β/ CCL19 | Il-6 | 0.39 | 0.005 |
| IL-10 | 0.40 | 0.002 | |
| Th17 Adaptive mediator | |||
| IL-17A | IL-1β | 0.38 | 0.006 |
| IL-6 | 0.29 | 0.041 | |
| IL-8 | 0.32 | 0.022 | |
The analysis of the cytokines and chemokines associated with Th1 and Th17 cell immune responses in sera of the 50 BB patients showed levels significantly higher of MIP-3β/CCL19 Th1 (p = 0.031) and IL-17A Th17 (p = 0.037) inflammatory mediators than the levels of healthy controls (Table 3). We could find the correlation between the MIP-3β/CCL19 Th1 and IL-6 innate cytokine (r = 0.39, p = 0.005), in addition to MIP-3β/CCL19 and IL-10 innate cytokine (r = 0.40, p = 0.002) described above. The IL-17A Th17 cytokine was correlated with the innate associated mediators IL-1β (r = 0.38, p = 0.006), IL-6 (r = 0.29, p = 0.041) and IL-8 (r = 0.32, p = 0.022).
DiscussionBrazilian Borreliosis is an infectious disease transmitted by ticks that is similar to Lyme disease (LD), the most commonly reported tick-born illness in Northern Hemisphere. In Brazil, despite the increasing number of suspected cases in all regions, this disease is still neglected by the medical communities. Since 1992, when the BB disease was described,1 it has been studied by multidisciplinary researchers in order to elucidate all aspects of this illness. The present study is the first to investigate the inflammatory response in BB disease. We analyzed the innate and Th1/ Th17 adaptive cytokines and chemokines in serum samples of acute and convalescent patients, since this disease may have a long latency period between initial infection or tick bite and onset of symptoms. All of the patients had a history of the erythema migrans at the site of tick bite and had received no antibiotic treatment. The evaluated serum samples were sent from different regions of the country, showing the large geographic distribution of the cases in Brazil. The demographic data showed a wide age range of patients, mainly in acute phase of disease. The same age range of BB patients was described previously by Gouveia et al.18 The majority of patients who met the BB diagnostic criteria was female, probably because they search for medical care more promptly. The patients developed a large spectrum of cutaneous, osteomuscular, neurological, cardiac and psychiatric disorders in acute and convalescent phases of illness. These clinical manifestations were described previously by Shinjo et al.19 and Rosa Neto et al.20 In our work, nearly half of the BB patients had positive IgM or IgG antibodies against Borrelia burgdorferi American G39/40 strain whole-cell sonicate antigen in all stages of illness. The low sensitivity of serology had been indicated previously and might have been biased in that the used antigen was not endemic, considering that the BB etiologic agent was not isolated from culture medium, although the bacteria from BB erythema migrans biopsy and blood samples were classified in the Borrelia burgdorferi senso lato complex.4,5 Heterogeneity in the sensitivity of enzyme-linked immunosorbent assays (ELISAs) was shown when different Borrelia spp. were used for serologic diagnosis of Lyme disease, suggesting the importance of autochthone antigens.21 The wide confidence interval for immune mediators investigated in this study is attributed to high sensitivity of the multiplex bead-based assay. In addition, the confidence intervals varied depending upon the specific cytokine or chemokine analyzed.22
The aim of this study was to characterize the innate and Th1/Th17 adaptive immune responses in patients with acute and convalescent BB patients. The analysis of the cytokines and chemokines involved in innate immune response showed that the levels of the IL-8 and MIP-1α/CCL3 chemokines were higher in serum samples of 50 BB patients compared to healthy controls. In addition, IL-1β, IL-8 and MIP-1α/CCL3 levels were significantly more elevated in serum of acute BB patients compared to healthy controls. In agreement with our results, Grygorczuk et al. reported that the chemokine ligand (CCL)-3, also known as macrophage inflammatory protein (MIP)-1α, and the chemokine IL-8 were elevated in serum samples of LD patients with erythema migrans (early localized infection), patients with neuroborreliosis (early disseminated infection) and patients with Lyme arthritis (chronic infection) before antibiotic treatment compared to samples of healthy controls.23 Interestingly, Borrelia burgdorferi genotypes isolated from erythema migrans skin lesions of LD patients stimulated macrophages to secrete the MIP-1α/CCL3 and IL-8 inflammatory mediators in vitro, reinforcing the important role of innate immunity in Borrelia burgdorferi infection.17,24 In addition, Borrelia burgdorferi was able to induce the synthesis of the IL-1β innate cytokine from human peripheral blood mononuclear cells.25 Mullegger et al. reported that the IL-1β was expressed in erythema migrans biopsies of early LD patients.26 In contrast, the levels of innate associated serum cytokines and chemokines of convalescent BB patients were not significantly different from the levels of healthy controls.
Our results suggest that the adaptive immune response also has an important role in BB disease beyond innate immunity. We demonstrated that CCL19 (also known as MIP3β) from Th1 adaptive immunity showed significantly higher levels in serum samples of BB patients than in healthy controls. Elevation of T cell chemoattractant CCL19, shown to be related to early LD, was described in serum samples of acute LD patients before treatment and was associated with the acute inflammatory mediators C-reactive protein (CRP) and serum amyloid A.12 In addition, this immune mediator seems to play a role in many phases of LD. In fact, Alcott et al. suggested that the chemokine CCL19 (Th1 adaptive immunity) could be considered an immunological marker of evolution to post-LD syndrome (PLDS), which occurs in approximately 20% of patients after treatment. PLDS is characterized by musculoskeletal pain and cognitive dysfunction after antibiotic treatment.14 We suggest that the cytokine associated with adaptive Th17 immune response also play a role in immunopathogenesis of BB disease. The levels of the IL-17A cytokine in serum samples of all BB patients were significantly higher than in healthy controls. IL-17 has been proposed to play a role in the pathogenesis of Lyme arthritis. Strle et al. showed robust IL-17A responses in synovial fluid of untreated LD patients compared with healthy subjects.27 In addition, Codolo et al. showed that synovial T cells extracted from patients with Lyme arthritis produced IL-17 in response to Borrelial antigen.15 Additionally, in an experimental mouse study, IL-17 inhibition prevented the development of Lyme arthritis.28
In this study, BB patients showed a wide range of symptoms in all phases of disease, suggesting the contribution of inflammatory mediators. Innate TNF-α was correlated with many clinical symptoms and disseminated infection. Mullegger et al. described greater expression of pro-inflammatory TNF-α mRNA in skin biopsy of erythema migrans and acrodermatitis chronica atrophicans. These lesions were associated with many clinical manifestations of acute and late LD.26 In addition, TNF-α is an innate immune cytokine expressed in serum and synovial membrane of patients with Lyme arthritis and in normal peripheral blood mononuclear cells (PBMCs) stimulated by Borrelia burgdorferi genotypes from LD patients.27 Bouquet et al. showed TNF-α gene expression by longitudinal transcriptome analysis from PBMCs of LD patients.29 Previous studies showed that the TNF-α cytokine, expressed in the presence of Borrelia sp., was involved in the pathogenesis of Lyme arthritis and autoimmunity symptoms.30,31
The correlation of immune mediators in BB patients suggests that the innate cytokines and chemokines may be interacting. Experiments have shown that IL-10 modulates inflammatory cytokines and chemokines produced by murine and human immune cells stimulated by live Borrelia burgdorferi or borrelial surface-exposed lipoprotein.32,33,34,35
ConclusionThe present study showed that BB patients display predominant innate immunity activity during the acute phase of disease. In addition, the Th1 and Th17 adaptive immunity also play a role in acute and convalescent phases. This study suggests that the TNF-α innate cytokine may be involved in the pathogenesis of clinical manifestations of BB disease. Further research regarding the role of the cells and other immune system mediators is needed to characterize the immune physiopathology of BB disease.
FundingFundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) # 2017/12778-7 (VLNB), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) # 305556/2017-7 (RMRP).
Conflicts of interestThe authors declare no conflicts of interest.