In Brazil the knowledge about methicillin-resistant Staphylococcus aureus infection in cystic fibrosis patients is scarce. This study aimed to determine the incidence of respiratory tract colonization and the identification rates after a standardized treatment. A retrospective cohort was performed highlighting the history of respiratory colonizations between January 2008 and June 2015. Patients under the age of 21 years with cystic fibrosis confirmed by sweat test or genetic study receiving care at the outpatient clinics of a Teaching Hospital were included. The treatment consisted of trimethoprim/sulfamethoxazole, rifampicin, nasal mupirocin and chlorhexidine 2%. The mean follow-up period was of 22.2 months and those with ≥3 negative cultures were considered free of methicillin-resistant Staphylococcus aureus. Forty-two patients were included. Methicillin-resistant Staphylococcus aureus was identified in six patients. Most patients had methicillin-sensitive S. aureus isolation prior to methicillin-resistant Staphylococcus aureus. Five children used the standardized treatment, none presented side effects. Only one child had a new isolation of methicillin-resistant Staphylococcus aureus during follow-up (after 20 months). The incidence of methicillin-resistant Staphylococcus aureus infection was high and occurred in young patients. The therapeutic regimen was effective, safe and being a good option to treat methicillin-resistant Staphylococcus aureus infection.
Cystic fibrosis (CF) clinical manifestations are highly variable and respiratory tract infections (RTI) are responsible for high morbidity and mortality rates. Identifying and better understanding the respiratory tract pathogens in those patients is therefore of utmost importance.1
Staphylococcus aureus is one of the first and most frequent pathogens to be isolated from the respiratory tract in CF patients.2 This microorganism is a Gram-positive coccus with a typically aerobic metabolism that can also perform as a facultative anaerobic and is capable of producing biofilm.2
In the last decades the increased life expectancy of CF patients can be related to advances in the treatment of chronic pulmonary infections and also to patient follow-up by multidisciplinary healthcare teams.1 The emergence of multi-resistant microorganisms is mainly a result of multiple antibiotic cycles in the treatment of RTI1 and to long-lasting hospitalizations.3
The isolation of methicillin-resistant S. aureus (MRSA) was first observed in 1961.2 Resistance is acquired through the mecA gene located on the Staphylococcal cassette chromosome mec complex (SCCmec).4 Based on its structural composition, 11 different types (I-XI) and various subtypes of SCCmec have been recognized in MRSA so far.3,4 Initially, MRSA infections were only described in the hospital environment. However, in the 90s MRSA began to be observed also in the community.2
The prevalence of MRSA colonization in CF patients has been increasing in the USA for the last 15 years,2,3,5,6 being variable in diverse geographic regions, ranging from 2.7% in the United Kingdom to 30% in the United States.2,3,7,8 There are doubts regarding the natural course and pathogenic importance of the infection caused by this microorganism, although it is possibly associated with a more rapid decrease in lung function and higher morbidity and mortality.9,10 The role of MRSA eradication treatment in order to prevent chronic infection is also unclear3 and there is no consensus about the ideal therapeutic regimen and various regimens are currently in use.2,3,11
In Brazil the knowledge about MRSA colonization in CF patients is scarce. A study carried out in 2006 in a reference center in Bahia identified that the frequency of MRSA colonization was 6%12 and Simon et al.13 detected a frequency of 18.8% in CF patients at a center in Porto Alegre. Data from the Brazilian Cystic Fibrosis Registry (BCFR) show a frequency of MRSA isolation varying from 7.8% to 9.3% between 2009 and 2013.14
This study aimed to determine the incidence of respiratory tract colonization by MRSA and to assess the persistence rates of the microorganism after administering a standardized treatment in CF pediatric patients followed at the Multidisciplinary Cystic Fibrosis Clinic at the Prof. Edgard Santos Teaching Hospital, Salvador, Brazil. This clinic provides treatment for children and adolescents (0–20 years old) mostly from low income families and with high level of miscegenation.
A retrospective cohort study was conducted searching for history of respiratory colonization among CF children and adolescents under 21 years of age followed at the Multidisciplinary Cystic Fibrosis Clinic at the Prof. Edgard Santos Teaching Hospital in the period of January 2008 and June 2015. The patients included in the study had a CF diagnosis confirmed by elevated sweat chloride tests on two different occasions.
Data used in this study were retrieved from patients’ medical charts and the following variables were collected: sex; ethnicity; current age, age at the diagnosis and age at the first isolation of methicillin-sensitive S. aureus (MSSA) and MRSA; number of MRSA-positive samples for each patient; previous MSSA identification; Pseudomonas aeruginosa prior to and/or after the MRSA isolation; genotype; use of standardized treatment.
Patients’ follow-up visits at the Multidisciplinary Cystic Fibrosis Clinic usually were scheduled every three months and collection of respiratory secretions by the physiotherapist is done at every visit. Samples were preferably obtained via expectorated sputum or by oropharyngeal swab when the former way was not possible and the material was immediately sent to the microbiology lab for culture. Upon arrival, samples were processed in blood agar, chocolate agar, MacConkey agar and selective medium for Burkholderia cepacia complex. Plates with creamy white hemolytic colonies, suggestive of S. aureus, were Gram stained and the phenotypic coagulase test was performed using Staphytest – PROBAC®. Gram-positive coagulase-positive samples were then submitted to an antimicrobial susceptibility test for the detection of MRSA using a cefoxitin 30g disk in Mueller–Hinton medium. Cefoxitin-resistant isolates were considered MRSA according to the Clinical and Laboratory Standards Institute (CLSI).15 The standard American Type Culture Collection (ATCC) S. aureus was used as a control for the quality of the medium and disks used.
All patients with MRSA isolation between January 2013 and August 2014 received a standardized treatment consisting of an oral association of trimethoprim/sulfamethoxazole plus rifampicin for 14 days, besides nasal mupirocin and corporal hygiene of the patient and family members with chlorhexidine 2%, both for one week. After treatment, patients were followed-up until June 2015 and those with at least three negative cultures during this period were considered free of MRSA.
Descriptive analysis was performed using Epidata software and the following descriptive statistics were calculated: median, mean, standard deviation, amplitude, simple and relative frequencies. The study was approved by the Internal Review Board of Prof. Edgard Santos Teaching Hospital under approval number 121/2011. Informed consent statements were signed by one of the parents, for patients under 18 years old, and, for the others, by the own patient.
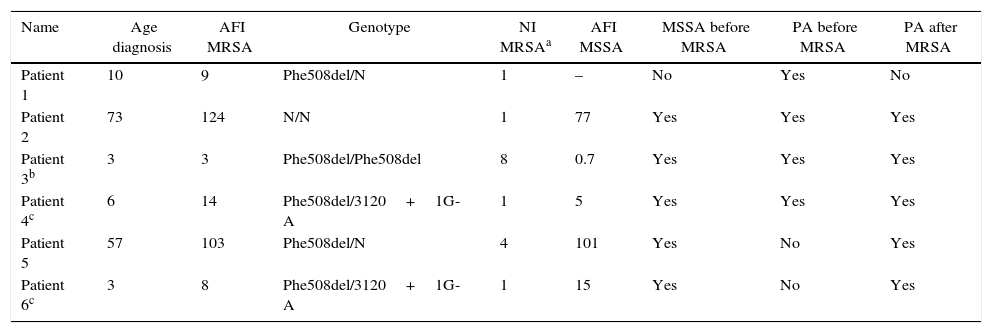
Overall, 42 patients were included in the study with a mean age of 9.9 years (SD=5.4) ranging from 1 to 20.8 years. From those, 22 (52.4%) patients were male. Eight patients followed at the cystic fibrosis Clinic were excluded: three had suppurative lung disease and chronic respiratory tract colonization by mucoid P. aeruginosa but normal sweat tests, and three had borderline sweat tests. All patients were non-white. The allelic frequency for the Phe508del mutation was 25%. During the study period, MRSA was identified in six patients (14.3%) and their clinical data are displayed on Table 1. Two children had more than one isolation of this microorganism (four and eight, respectively). The sensitivity profiles of this microorganism showed susceptibility to trimethoprim/sulfamethoxazole and vancomycin, among other tested antibiotics.
Clinical data from the six patients with MRSA isolation from 2011 to 2014.
| Name | Age diagnosis | AFI MRSA | Genotype | NI MRSAa | AFI MSSA | MSSA before MRSA | PA before MRSA | PA after MRSA |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 10 | 9 | Phe508del/N | 1 | – | No | Yes | No |
| Patient 2 | 73 | 124 | N/N | 1 | 77 | Yes | Yes | Yes |
| Patient 3b | 3 | 3 | Phe508del/Phe508del | 8 | 0.7 | Yes | Yes | Yes |
| Patient 4c | 6 | 14 | Phe508del/3120+1G-A | 1 | 5 | Yes | Yes | Yes |
| Patient 5 | 57 | 103 | Phe508del/N | 4 | 101 | Yes | No | Yes |
| Patient 6c | 3 | 8 | Phe508del/3120+1G-A | 1 | 15 | Yes | No | Yes |
Age at the diagnosis (in months).
AFI, age at the first isolation (in months); NI, number of isolations; N, normal allele for the six tested mutations; MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus; PA, Pseudomonas aeruginosa.
In the six patients in whom MRSA was isolated, the median age at CF diagnosis was 8 months, varying from three to 73 months. The median age at the first MRSA identification was 11.5 months, ranging from three to 124 months, and the median age at the first MSSA isolation was 15 months, which varied from 0.7 to 101 months. Most patients (83.3%) presented at least one MSSA isolation prior to MRSA detection, and one P. aeruginosa identification prior to (66.7%) and post (83.3%) MRSA detection.
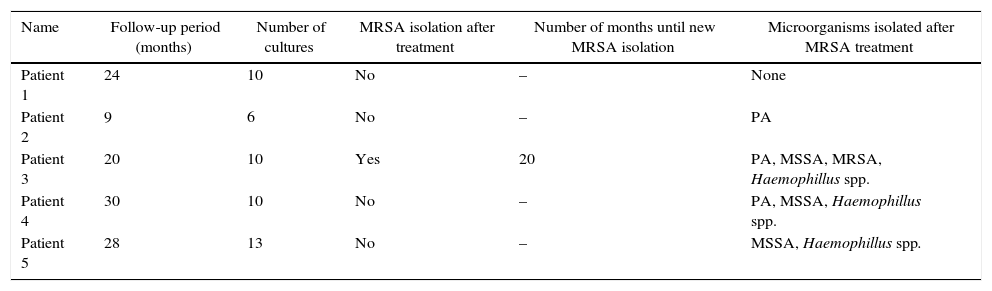
Five children with MRSA isolated after 2013 were treated with the above described regimen and none of them presented side effects during the period of the study. Respiratory secretions were collected in at least three occasions during six months after treatment. All children were young and/or non-suppurative so all microbiological samples were collected using oropharyngeal swab. Only the child with eight MRSA isolations (including one in blood culture) had a new isolation during the follow-up period. Table 2 presents data of the respiratory specimens studied during follow-up.
Respiratory specimens studied during follow-up of the five children that used the standardized treatment.
| Name | Follow-up period (months) | Number of cultures | MRSA isolation after treatment | Number of months until new MRSA isolation | Microorganisms isolated after MRSA treatment |
|---|---|---|---|---|---|
| Patient 1 | 24 | 10 | No | – | None |
| Patient 2 | 9 | 6 | No | – | PA |
| Patient 3 | 20 | 10 | Yes | 20 | PA, MSSA, MRSA, Haemophillus spp. |
| Patient 4 | 30 | 10 | No | – | PA, MSSA, Haemophillus spp. |
| Patient 5 | 28 | 13 | No | – | MSSA, Haemophillus spp. |
PA, P. aeruginosa; MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus.
The incidence of MRSA colonization in this study (14.3%) was higher than that reported in a previous study conducted in Salvador12 and in the BCFR between 2009 and 2013,14 but lower than the rate found by Simon et al.13 It is important to highlight that our study sample was consisted mostly of younger children and there were limitations in the collection of the respiratory secretions; thus, the frequency of isolation of this microorganism could have been underestimated.
The median age at the first MRSA colonization in the present study was 11.5 months (3–124) evidencing a very early identification of this microorganism since 50% of the children had their first isolation before the first year of life, which is in accordance with the study conducted by Salsgiver et al.6 that demonstrated higher incidence of MRSA detection in this age group. It is also possible that the socioeconomic characteristics of our population contribute to a higher occurrence of MRSA, as suggested by David et al.16
P. aeruginosa was identified in 4 out of 6 patients (66.7%) and 5 out of 6 patients (83.3%) prior to and after MRSA isolation, respectively. The use of multiple antibiotics could have predisposed isolation of new pathogens.17
It is important to highlight that the allelic frequency for the Phe508del was of 25% in the studied population, which differs from much higher figures observed in studies with Caucasian populations.18,19 However, most MRSA colonized patients (83.3%) presented this mutation, being heterozygous in four of those, and homozygous in one patient, with allelic frequency of 50%, suggesting an association with a genotype related to a more severe disease, which is a risk factor for MRSA infection. Similarly to that, Al-Zubeidi et al.20 observed that 93% of the patients with positive culture for MRSA presented this mutation and most of them were homozygous (70%).
During the follow-up period only one child had a new MRSA isolation after being treated with the standardized regimen. It is important to emphasize that there was a 20-month delay for a positive culture in this patient. There is no consensus about the ideal treatment for MRSA infection. Various regimens have been proposed, with different means of administration and duration. However, there are few controlled studies with the evaluation of possible side effects.2,11 The treatment regimen used in this study demonstrated to be effective, besides having the advantages of being oral, with comfortable dosage and the possibility of being used in early ages for a brief period of time. Nonetheless, randomized studies should be conducted, with larger sample sizes and careful side-effects monitoring.
Among the limitations of this study are the small sample size, consisting only of children and adolescents, selected at only one reference center, and the retrospective data collection. Besides that, included patients were mostly young and non-suppurative children, which limited the collection of expectorated sputum for the isolation of microorganisms, a technique that has higher sensitivity when compared to oropharyngeal swab. More sensitive techniques such as bronchoalveolar lavage were not used, nor the determination of MRSA genotype. Therefore, it was impossible to identify the strains and the most common types and subtypes found, which could have helped to determine if MRSA was hospital or community acquired. Notwithstanding, the susceptibility profiles identified suggest that microorganisms found were community acquired. The follow-up time and age group studied did not allow us to rule out any late outcomes. On the other hand, the results allowed us to know the incidence of MRSA in the group of children with CF in this multidisciplinary center and suggested that the treatment used was effective in reducing the rates of MRSA identification while also making possible the adoption of measures to control cross-infection between patients.
There was a high incidence of MRSA in the studied population. The treatment used was effective and safe and should be considered as a therapeutic option for this patient population. Considering these results and the influence of this pathogen in the outcomes of pulmonary disease in CF we highlight the importance of performing controlled studies with extended number of patients so that effective MRSA eradication strategies can be developed.
FundingThis study was supported by Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB). The funding source did not participate in any steps of the study including planning, data collection, analysis or interpretation of the data. Also the funding source did not influence the decision to submit the paper to publication.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the cystic fibrosis patients and their families for allowing this study to be conducted. In addition, they are grateful to the professionals at the multidisciplinary outpatient clinic of the Federal University of Bahia's Teaching Hospital for providing patient care.