Objective: To determine the incidence of surgical site infection in patients undergoing craniotomy and to compare 12-month and 3-month post-discharge surveillance periods in terms of their impact on the incidence of surgical site infection in those patients.
Methods: This was a retrospective cohort study involving 173 adult patients submitted to “clean” craniotomy, with or without implants, during the six-month period, at a university hospital in the city of São Paulo, Brazil. All the patients were evaluated in the pre-, trans- and postoperative periods and were followed for 12 months to analyze the development of surgical site infections.
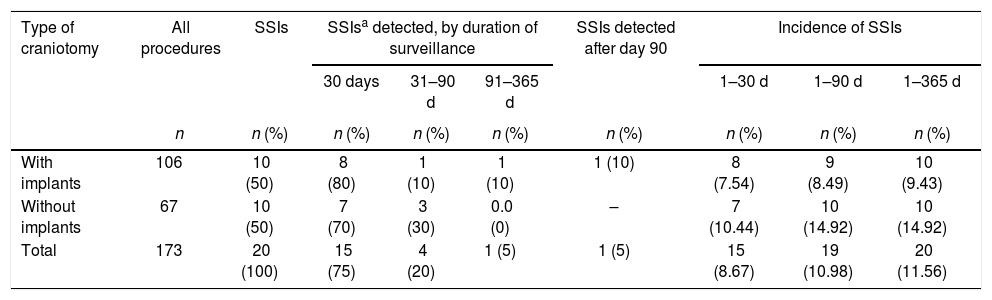
Results: Of the 173 patients undergoing craniotomy during the study period, 20 developed an surgical site infection during the first, and 12 months after discharge, the overall incidence of surgical site infection therefore being 11.56%, compared with a 1-month incidence of 8.67% and a 3-month incidence of 10.98%. Among the 106 patients who received implants, the 1-, 3-, and 12-month incidence of surgical site infection was 7.54% (n = 8), 8.49% (n = 9), and 9.43% (n = 10), respectively. Among the 67 patients who did not receive implants, the 1-, 3-, and 12-month incidence of surgical site infection was 10.44% (n = 7), 14.92% (n = 10), and 14.92% (n = 10), respectively.
Conclusion: The incidence of surgical site infection after craniotomy is high. Reducing the duration of the post-discharge surveillance period from 12 months to 3 months did not cause significant losses in the numbers of surgical site infection identified or a substantial decrease in their incidence.
Surgical site infection (SSI) is considered the most common health care-associated infection, accounting for 31% of all infections in hospitalized patients.1 Alongside an associated mortality rate of 3%, SSIs increase both mortality and morbidity rates, thereby increasing hospital stays by 7–10 days, with an estimated increase of $1 billion (in 2009 U.S. dollars) in annual cost of treatment.2,3
In Brazil, SSI is one of the main health care-associated infections, ranking third among all infections occurring at health care facilities and accounting for 14–16% of all infections in hospitalized patients. A nationwide study conducted in 1999 by the Brazilian Ministry of Health found an SSI rate of 11% among the surgical procedures evaluated.4
In 2013, Rosenthal et al.5 published the International Nosocomial Infection Control Consortium report, showing surveillance data for the 2005–2010 period in 30 countries, 10 of which were Latin-American countries, including Brazil, where the reported SSI rate was 1.6%. Infections occurring after neurosurgical procedures, especially craniotomies, directly affect the prognosis, increasing morbidity and mortality, as well as lengthening hospital stays and increasing the reoperation rate.6 The reported incidence of SSI after neurological procedures is 5% on average, ranging from 1% to 11%.7 A study conducted in hospitals in the United States by the Centers for Disease Control and Prevention, in cooperation with the National Healthcare Safety Network,8 the reported post-craniotomy SSI rate for the 2006–2008 period was 1.3%. By comparison, a rate of 4.4% was reported in Latin American hospitals in a study conducted by the International Nosocomial Infection Control Consortium (p = 0.0001).5
The recommended period of post-discharge surveillance for SSIs differs between the previous guidelines (issued in 2008) and those currently in effect. The 2008 guidelines recommended that, for procedures involving the use of implants or prostheses, such surveillance be maintained for 12 months, whereas the recommended duration of surveillance for procedures not involving the use of implants or prostheses was only one month. The current guidelines, issued in 2014, state that the duration of post-discharge surveillance for SSIs should be determined on the basis of the type of surgical procedure and the topography.9 The list of procedures for which three-month post-discharge surveillance is recommended includes some types of neurosurgery, such as craniotomy, spinal fusion, and ventricular shunt. For all types of procedures, the new guidelines recommend only one month post-discharge surveillance for superficial SSIs.8
In this study, we compared the incidence of SSI at three and 12 months after craniotomy with and without implants, with the objective of analyzing the impact reduction in duration of surveillance would have on incidence. The new guidelines recommend a 3-month period of surveillance for deep incisional and organ–space SSIs after craniotomy. However, craniotomy patients evaluated in the present study, screening for such infection, were followed for 12 months. Therefore, the proportion of SSIs that would have been missed had the patients not been followed for the additional nine months could be determined.
Material and methodsThis was a retrospective cohort study, conducted at the Hospital São Paulo, a university hospital operated by the Federal University of São Paulo, in the city of São Paulo, Brazil. We included consecutive patients who underwent craniotomy between January 1 and June 30, 2014, excluding only those who did not meet the study criteria.
During the study period, 413 neurosurgical procedures were performed at Hospital São Paulo. Of those, 341 were craniotomies. Patients under 18 years of age were excluded, as were those who underwent craniotomy in the presence of existing infection, those who presented with signs of SSI, those who underwent surgery that was potentially contaminated, contaminated, or infected, and those for whom there were missing data in the patient charts. The final cohort comprised 173 patients.
Surveillance for SSI for patients included in the study was maintained for a period of 12 months (365 days) after the surgical procedure, in order to assess the incidence of SSI. The patients’ charts were analyzed prospectively. We also collected data regarding subsequent hospitalizations and outpatient follow-up visits at the same hospital, in order to detect evidence of SSI.
SSI was diagnosed based on criteria for health care-associated infections defined at the current Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance guidelines.8 According to these guidelines, the recommended post-discharge surveillance period for deep incisional and organ–space SSIs after craniotomy is three months.
The craniotomy patients were divided into two groups according to the occurrence of SSI diagnosed in the different periods of surveillance. We also evaluated those who had received implants separately from those who had not. For all patients, we compared the currently recommended post-discharge SSI surveillance period (three months) with two other periods: one month and 12 months. We defined implants as orthotics or prostheses, whether total or partial and whether implanted permanently or transiently, including derivations with internal or external terminal portions (ventriculoperitoneal shunt or external ventricular drain, respectively), electrodes, vascular clamping devices, cranial reconstructive material, and osteosynthesis material.
For all 173 patient charts, we used a standardized instrument designed to verify the data related to the preoperative, intraoperative, and postoperative processes. Information regarding outpatient follow-up and subsequent hospitalizations was obtained from the electronic health record system of the institution.
Statistical analysisVariables of interest were initially compared between patients with and without SSI using the chi-square test (or Fisher’s exact test) for categorical variables and the t-test for quantitative variables.
Variables associated with SSI with a level of significance of up to 10% (p ≤ 0.10) were included in the initial logistic regression model. Through the backward selection process, the least significant variables were removed from the model, until a final model with only those significantly associated with SSI (p < 0.05) was obtained. The data were tabulated in an Excel spreadsheet and analyzed with Minitab statistical software, version 16.1 (Minitab Inc., State College, PA, USA). Values of p < 0.05 were considered statistically significant.
ResultsOf the 173 patients undergoing craniotomy during the study period, 20 developed an SSI during the first 12 months after discharge with an overall incidence of SSI of 11.56%. Of the 20 SSIs identified, 13 (65%) were organ-space infections, 4 (20%) were superficial incisional infections, and 3 (15%) were deep incisional infections. The microorganism most often found at the surgical site was Staphylococcus aureus, which was isolated in three cases (15%).
The mean time from craniotomy to the diagnosis of SSI was 34 ± 48.9 days. The mean age of the 173 patients was 51.9 ± 17.2 years (range, 18–87 years), and 88 (50.9%) were male. The mean age of the patients with and without SSI was 45.2 years and 52.9 years, respectively, and the difference was statistically significant (p = 0.043). No significant statistical difference was observed between the groups with and without infection, in relation to the comorbidities evaluated. Our study showed that 95 (55%) patients in the sample presented a Charlson score ranging from 1 to 2. However, the group of patients with SSI had a higher score when compared to the non-infected group, 13 (65%) versus 82 (54%), respectively. The antimicrobial prophylaxis, as well as the compliance with the administration schedule (up to one hour prior to the incision) and duration of prophylaxis, were not significant when comparing patients with and without SSI (p = 0.311, p = 0.599, p = 0.621, respectively).
Comparing the patients with and without SSI, we found no significant gender-based difference (p = 0.069).
The use of implants was not significantly different between the craniotomy patients with and without SSI (p = 0.271).
In the multivariate analysis, the following variables were identified as independent risk factors for SSI: surgical time greater than four hours (OR, 1.49; 95% CI, 0.96–2.30; p = 0.076), drainage time (OR, 4.67; 95% CI, 1.34–16.00; p = 0.015), and having undergone at least one reoperation (OR, 4.38; 95% CI, 1.14–16.74; p = 0.03).
The incidence of SSI was compared among the three periods of post-discharge surveillance. Out of the 173 patients of the whole cohort, SSIs occurred during the 1-, 3-, and 12-month surveillance periods in 15, 19, and 20 patients, respectively, with incidence rates being 8.67%, 10.98%, and 11.56%. Among the 106 patients who underwent craniotomy with an implant, SSIs occurred during the 1-, 3-, and 12-month surveillance periods in 8, 9, and 10 patients, respectively, corresponding to incidence rates of 7.54%, 8.49%, and 9.43%. Therefore, reducing the post-discharge surveillance period from 12 months to three months would have missed one (10%) SSI in the group of patients who received an implant, reducing the 12-month incidence of SSI by 0.94% in that group and by 0.58% in the whole cohort. For the group of patients who underwent craniotomy with no implant and subsequently developed an SSI, reducing the post-discharge surveillance period from 12 months to three months would not have resulted in any SSI going undetected. However, if the duration of post-discharge surveillance for SSIs were further reduced from three months to the previously recommended one month, three (30%) of the 10 SSIs identified in the no implant group would have gone undetected. Including those cases increased the 3-month SSI incidence by 4.48% in that group (Table 1). In the group of patients who received implants, surveillance had greater sensitivity for the detection of an SSI when maintained for 12 months rather than three months or one month (100% vs. 90% and 80%, respectively), although the positive predictive value was the same (100%) for all three surveillance periods.
Incidence rates of surgical site infection after craniotomy, with or without implants, according to duration of surveillance. Hospital São Paulo, January 1 through June 30, 2014.
| Type of craniotomy | All procedures | SSIs | SSIsa detected, by duration of surveillance | SSIs detected after day 90 | Incidence of SSIs | ||||
|---|---|---|---|---|---|---|---|---|---|
| 30 days | 31–90 d | 91–365 d | 1–30 d | 1–90 d | 1–365 d | ||||
| n | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| With implants | 106 | 10 (50) | 8 (80) | 1 (10) | 1 (10) | 1 (10) | 8 (7.54) | 9 (8.49) | 10 (9.43) |
| Without implants | 67 | 10 (50) | 7 (70) | 3 (30) | 0.0 (0) | – | 7 (10.44) | 10 (14.92) | 10 (14.92) |
| Total | 173 | 20 (100) | 15 (75) | 4 (20) | 1 (5) | 1 (5) | 15 (8.67) | 19 (10.98) | 20 (11.56) |
Compared to the incidence of post-craniotomy SSIs reported in the literature, the rate observed in the present study is high (11.56%), especially because only “clean” non-traumatic craniotomies (without previous contamination of the surgical site) were considered. In a study of more than 2000 neurological procedures, 1587 of which involved cranial access, 14 patients developed an SSI, with an incidence of 0.8%.10
The incidence of post-craniotomy SSI varies substantially across studies, which might reflect differences among patient populations, types of procedures, and types of infections evaluated.11 According to the National Healthcare Safety Network report issued by the Centers for Disease Control and Prevention,12 the incidence of post-craniotomy SSI in the United States ranged from 2.15% to 4.66% in the 2006–2008 period.
Among the 20 SSIs diagnosed in our study, the most common type of infection was meningitis, which accounted for 10 (50.0%) infections overall and for 76.9% of the 13 organ-space infections. In our cohort, deep incisional and organ-space infections accounted for 80% of all SSIs diagnosed, which is in keeping with the findings of previous studies showing that SSIs of greater severity are also those for which the prevalence is higher.13 The predominance of S. aureus among the SSIs diagnosed in our cohort is in agreement with the results of various studies in the literature, suggesting contamination from the skin.14
In the present study, the mean time from the surgical procedure to the diagnosis of an SSI was 34 days, 19 (95%) out of 20 SSIs having been diagnosed within the first three months after discharge. In two separate studies, Miyake et al.15 and Bellusse et al.16 reported mean times from surgery to diagnosis of an SSI of 23.3 days and 12.8 days, respectively, both lower than that identified in our study.
Bellusse et al.16 reported a mean patient age similar to the 51.9 years in our cohort. In that study, conducted in 2013, there was also a slight predominance of males, who accounted for 57.6% of the sample, comparable to the 50.9% in our study cohort.
The most prevalent comorbidities in the present study were: cerebrovascular disease (80% in the SSI group, 75% in the non-SSI group), systemic arterial hypertension (50% in the SSI group, 48% in the non-SSI group), presence of neoplasia (28% in the group with SSI, 34% in the non-SSI group), smoking (17% in the SSI group, 22% in the non-SSI group), diabetes mellitus (6% in the SSI group, 23% in the SSI group, 15% in the non-SSI group). Previous studies have indicated that patients with systemic disease have a higher incidence of SSI and also demonstrated a direct relationship between clinical severity and the occurrence of SSI.17
For antimicrobial prophylaxis, the most commonly used antimicrobial agent was cefazolin (95; 54.91%), followed by cefuroxime (34; 19.65%), vancomycin and ceftriaxone (19; 10.98%), and ceftriaxone (4; 2.31%). For the group of patients who developed SSI, cefazolin (10; 50%) was the most used, followed by cefuroxime (4; 20%) and vancomycin and ceftriaxone (4; 20%). No patient in the infection group used ceftriaxone (0%).
In this study, the decision to perform surveillance of SSIs for one year was motivated by the current definition of CDC surveillance issued in 2014, which limited the duration of the surveillance period for craniotomies with implants at 90 days. Our intention was to compare the number of SSIs detected according the old criterion (360 days) with the new criterion (90 days) and thus quantify and analyze the missed SSIs.
When we compared the various durations of surveillance in terms of the proportion of SSIs detected, we found that, of the 10 SSIs diagnosed among the patients who had undergone craniotomy with implants, only one (10%) was detected between three and 12 months after discharge (i.e., during the additional nine months of surveillance), whereas, of the 10 SSIs diagnosed among the patients who had undergone craniotomy without implants, all were detected within the first three months after discharge. Most of the SSIs (8; 80%) diagnosed in patients who had received implants and seven (70%) of those diagnosed in patients who had not were detected within the first month after discharge. If we had maintained the surveillance for post-craniotomy SSI for a period of only one month, as recommended in the previous (2008) guidelines, three (30%) SSIs diagnosed in the group of patients who had not received implants would have been missed, because they were detected between 31 and 90 days after discharge. Therefore, the change in the recommended duration of surveillance, from one month to three months (2014 guidelines), improved SSI reporting in that group of patients. In a study conducted in 2015, Koek et al.18 also compared and evaluated the impact that reducing duration of surveillance could have on the incidence of SSI over a 10-year period among 105,607 surgical procedures, with and without implants. In that study, the surgical procedures involving implants were total hip arthroplasty and knee arthroplasty, whereas those not involving implants were mastectomy, colon resection, laparoscopic cholecystectomy, and cesarean section. The authors found that, if the surveillance period were reduced from 12 to three months, the proportion of SSIs missed would be 6% after total hip replacement and 14% after knee arthroplasty. Nonetheless, the authors suggested that a 9-month reduction in the surveillance period might not result in a substantial decrease in the incidence of SSIs. Although those authors evaluated a larger patient sample and different types of procedures, their findings are in agreement with ours. Among our patients who had undergone craniotomy with implants, the proportion of SSIs that would have been missed (i.e., those occurring between the third and twelfth month after discharge) was 10%, comparable to the 6–14% reported by Koek et al.18
ConclusionsIn our cohort of patients, a post-discharge surveillance period of three months was sufficient to detect the majority (95%) of post-craniotomy SSIs, the remaining 5% being detected between 91 and 365 days after discharge. In addition, 75% of the SSIs diagnosed in our cohort were detected within the first month after discharge.
Therefore, reducing the post-discharge surveillance period from 12 to three months did not result in a significant reduction in the incidence of SSI. However, prolonged surveillance may detect more cases of SSI.
Conflicts of interestThe authors declare no conflicts of interest.