Various leptospiral components have been identified and shown to be involved in tissue destruction. In addition, immune responses to leptospires have been implicated in target organ damages in severe leptospirosis cases. Several inflammatory mediators were shown to be higher in susceptible animals than in resistant hosts. Moreover, cytokines/chemokines and serum proteins induced following Leptospira infection were suggested to be biomarkers for disease severity in human leptospirosis. This review focuses on the role of immune responses in the severity of leptospirosis. Studies in both animal models and humans are discussed.
Leptospirosis is a worldwide zoonosis caused by spirochetes of genus Leptospira. Leptospirosis patients present with diverse clinical manifestations ranging from asymptomatic infection, mild infection with fever, headache, myalgia, dyspnea, to severe presentations involving multiple organs. Severe leptospirosis is characterized by jaundice, renal and hepatic failure.1–3 In addition, pulmonary hemorrhage has been increasingly shown to be a cause of death of leptospirosis patients.4 Although the causative agent of leptospirosis was discovered since early 19th century,5 the pathogenetic mechanisms of this infectious disease are still not clearly understood. Several components of pathogenic Leptospira have been identified.6–12 However, it is still not known why patients infected with pathogenic Leptospira present with diverse clinical manifestations.
The immune response to Leptospira is suggested to play a role in organ damages observed in Leptospira infection. Various inflammatory mediators and serum proteins were thought to be promising biomarkers for disease severity prediction. Several studies elicited immune response to leptospira by stimulating cells with Leptospira or Leptospira components in vitro or by injecting Leptospira components into animal models.13–16 Although these experiments could elicit an immune response, these approaches may not mimic infection-induced of immune response. This review focuses mainly on studies of immune response induced by Leptospira infection. Studies conducted in animal models and in humans are reviewed.
To demonstrate the role of immune response in organ damage observed in infectious diseases, production of inflammatory cytokines, chemokines and adhesion molecules are commonly investigated. These mediators have also been suggested to play role in leptospirosis pathogenesis.
Immune responses in Leptospira infected animal modelsHamsters and guinea pigs are commonly used as susceptible animal models for leptospirosis. Pathologies and bacterial burden in target organs following Leptospira infection were evaluated in these susceptible animals. Cytokine gene expression in blood from recovered and dead hamsters infected with leptospires has been reported.17 TNF-α, IL-10, IL-1α and COX-2 expression was significantly higher in dead hamsters than in the survivors. In addition, the expression of these cytokines was also higher in hamsters infected with virulent leptospires than in hamsters infected with an avirulent strain.
Mice are known to resist to Leptospira infection. Comparison of inflammatory mediators in hamsters and Oncins France 1 (OF1) resistant mice following Leptospira infection was reported. Infected hamsters demonstrated pathologies and bacterial burden in various organs. OF1 mice showed rapid Leptospira clearance. Cytokine/chemokine gene expression was assessed in this study. Compared to resistant mice, hamsters showed delayed and massive expression of IL-1β, IL-6, TNF-α, cyclo-oxygenase -2, IP-10/CXCL10 and MIP-1α/CCL3 in infected organs. In addition, IL-10, an anti-inflammatory cytokine, was induced faster in infected resistant mice than in infected hamsters.18
Although mice are resistant to Leptospira infection, C3H/HeJ, the TLR4 defective mice, have been shown to be susceptible to Leptospira infection.19 Immune response induced by leptospires in resistant (BALB/C) and susceptible (C3H/HeJ) mice were compared.20 Expressions of TNF-α and MIP-2/CXCL-2 in lungs, kidneys and livers of infected animals were determined by qPCR and ELISA. Expressions of TNF-α and CXCL-2 were delayed in C3H/HeJ mice. The sustained MCP-1 and IL-8 expression in lungs of infected C3H/HeJ was also shown.
The alteration of epithelial sodium channel (ENac) and Na-K-2Cl (NKCC) co-transporter expression in lungs of hamsters infected with Leptospira was reported. ENac expression was decreased whereas the expression of NKCC was increased in pulmonary cells of infected hamsters. This study suggested that alteration of ion channel following Leptospira infection may impair pulmonary function resulting in pulmonary damage observed in leptospirosis.21
It has been shown in vitro that TNF-α decreased ENac and IL-1 increased Na-K-2Cl co-transporter.22–25 As mentioned earlier, TNF-α and IL-1 were increased in Leptospira infection. These data suggest that these two cytokines could be involved in alteration of ion channels resulting in pulmonary damage.
Expression of TNF-α, TGF-β, IP-10 and IL-10 in kidneys of hamsters infected with Leptospira has been reported.26 However, correlation of these cytokine expression and kidney damage is still not clearly demonstrated.
Immune responses in leptospirosis patientsVarious studies demonstrated immune responses in human leptospirosis. Levels of T-cell chemoattractants, IP-10/CXCL10 and Mig/CXCL9 were higher in leptospirosis patients than in controls.27 However, correlation of these chemokines with disease severity was not shown. The level of IL-8 was investigated in leptospiral hepatitis. IL-8 levels were significantly higher in leptospirosis patients than in controls and severe symptoms were also associated with high IL-8 levels.28
Tajiki and Salomao in 1996 measured plasma level of TNF-α in 18 leptospirosis patients. They found TNF-α level to be related to kidney, liver and lung involvement.29 This study suggested that TNF-α level could be a useful marker for poor prognosis. In addition, the ratio of plasma IL-10/TNF-α was determined in leptospirosis patients with and without organ involvement. In this study, a high IL-10/TNF-α ratio was associated with less disease severity.30 In contrast, Kyriakidis et al. in 2011 investigated cytokine levels in 28 leptospirosis patients and found a high IL-10/TNFα ratio to be associated with fatal outcome.31
Expression of ICAM-1 (intercellular adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1) and C3a receptor on lung tissues of leptospirosis patients was studied. Expression of ICAM-1, VCAM-1 and C3a receptor on alveolar septa of patients who died from leptospirosis with pulmonary involvement was higher than in control groups.32 The expression of these adhesion molecules and complement receptor could promote leukocyte recruitment to infected tissues.
Levels of other serum proteins such as ST2, long pentraxin-3 (PTX3) and copeptins have been investigated in leptospirosis patients. ST2 is a member of interleukin receptor family and a receptor for IL-33. Two ST2 isoforms including membrane (ST2L) and soluble ST2 (sST2) have been identified. Plasma ST2 level is increased in heart and inflammatory diseases.33–36 PTX3 is an acute phase protein belonging to the pentraxin family. It is a mediator of inflammation and its expression is induced in various cell types.37 Plasma PTX3 was shown to be a severity marker of inflammatory conditions and cardiovascular diseases.38–41 Copeptin is a 39-amino acid peptide released from arginine vasopressin (AVP) during AVP processing. It has been suggested to be a biomarker for various conditions including bacterial sepsis.42–44
Wagenaar et al. determined soluble ST2 (sST2), TNF-α, IL-1β, IL-6, IL-8 and IL-10 levels in 68 severe leptospirosis patients. This study suggested that sST2, IL-6 and IL-8 levels were higher in non-survivors than in survivors. In addition, sST2 level was associated with bleeding and suggested to be a tissue damage marker. In another study, IL-6, IL-8 and long pentraxin-3 (PTX3) levels in 52 leptospirosis patients were investigated. The mortality rate of this group of patients was 27%. This study demonstrated that PTX3, IL-6 and IL-8 levels were associated with disease severity and mortality.45,46
Plasma copeptin level in leptospirosis patients has been suggested to be a predictor of survival. Among survivors, copeptin level decreased to baseline level within seven days.47 Serum nitrite has also been shown to be increased in leptospirosis compared to healthy controls. However, correlation of serum nitrite with disease severity was not shown in this study.48
DiscussionIncreasing evidence indicate that immune response could enhance tissue damage observed in leptospirosis patients, especially in severe cases. As described above, both inflammatory and anti-inflammatory cytokines were induced by Leptospira infection. Moreover, chemokines participating in neutrophil and mononuclear cell recruitment were also produced.
TNF-α is a cytokine widely studied in immune-mediated diseases and is the most investigated cytokine in leptospirosis.20,31,49 It is an inflammatory cytokine produced following toll-like receptor (TLR) stimulation.50 C3H/HeJ mice are TLR-4 defective and were shown to be susceptible to Leptospira infection.19 However, correlation of TLR-4 mutation and leptospirosis pathogenesis is not clearly established.
Compared to infected BALB/C mice, TNF-α production in Leptospira infected C3H/HeJ mice was delayed in one study.21 Similar result was observed when TNF-α gene expression was investigated in mice and hamsters infected with Leptospira. Experiment in C57BL/6 mice with knockout TNF-α receptors (TNFR) has been reported. Severe renal inflammation was observed in convalescent TNFR deficient mice. This study supported the role of rapid induction of TNF-α following Leptospira infection.51 In addition, TNF-α induction was shown to be higher in hamsters that did not die after experimental leptospiral infection.17
These data suggest that for bacterial eradication, inflammatory cytokines should be induced rapidly. Delayed expression may allow for bacterial colonization and multiplication. However, production of inflammatory cytokines should be properly regulated. Prolonged production could promote further tissue damage. Leptospira toxins could directly induce tissue damage which is further promoted by inflammatory response.
High IL-10/TNF-α ratio was shown to be associated with less disease severity in one study.30 However, in another study, high IL-10/TNF-α ratio was associated with fatal outcome of leptospirosis patients.31 These data suggested the role of both inflammatory and anti-inflammatory cytokines in disease outcomes of leptospirosis. However, application of these two cytokines ratio as a disease marker needs to be further investigated in larger groups of patients.
IL-10 level was induced faster in infected BALB/C than in infected hamsters.18 The rapid production of this anti-inflammatory cytokine could regulate inflammatory cytokines production resulting in alleviation of tissue damage. Besides promoting inflammation, TNF-α could be involved in impairment of pulmonary function by altering epithelium sodium channel.21,22 Role of TNF-α in sodium channel change in leptospirosis with pulmonary hemorrhage has not been directly reported.
Chemokine involvement has been widely investigated in leptospirosis. Induction of IL-8 was delayed and sustained in C3H/HeJ mice infected with leptospires than in the resistant mice.52 The association of IL-8 level and disease severity and mortality has been shown in leptospirosis patients.28
In addition to cytokines and chemokines, other serum inflammatory mediators including sST2, PTX3 and copeptin were investigated whether they could be used as biomarkers for leptospirosis severity.
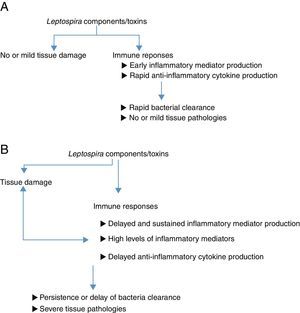
ConclusionFig. 1A and B are diagrams suggesting how host inflammatory responses participate in a wide range of leptospirosis symptoms. Various studies have shown that cytokines and serum proteins are promising biomarkers for leptospirosis severity. However, further studies should be done to confirm the reliability of these proteins in disease prediction. It would be interesting to investigate the levels of these suggested biomarkers in larger groups of patients with different degrees of leptospirosis illnesses. In addition, detection of inflammatory mediators should be performed using samples collected at various times after infection. The data obtained could confirm whether rapid or delayed production of inflammatory/anti-inflammatory mediators influences disease outcomes. Moreover, most studies investigated cytokines/chemokines levels in patient sera. In order to demonstrate whether immune responses participate in organ damage in leptospirosis, investigation of cytokines/chemokines levels in target organs should provide more convincing evidence.
Diagrams suggesting how inflammatory responses involve in different disease outcomes of leptospirosis. (A) Leptospira infection in resistant animal models or in hosts with mild symptoms. Leptospira components or toxins could directly induce tissue damage. Infected hosts recognize Leptospira and respond aiming at eradicating these infectious agents. Inflammatory responses occur early and rapidly to eradicate organisms. Further tissue dissemination is prevented. Anti-inflammatory cytokines are produced to regulate inflammation. Finally, leptospires are eradicated and no or mild tissue damage is observed. (B) Leptospira infection in susceptible animal models or in hosts with severe symptoms. Leptospira components or toxins could directly induce tissue damage. There is no clear evidence demonstrating that different pathogenic Leptospira contain different virulence factors. Infected hosts recognize Leptospira and respond aiming at eradicating these infectious agents. However, in these hosts, inflammatory responses were delayed. Organisms persist and promote further inflammatory responses. The delay of Leptospira eradication may provide time for bacterial dissemination to various organs. These prolonged and massive immune response could promote further tissue damages resulting in severe organ damage observed in susceptible animals or hosts with severe clinical manifestations.
Genetic difference could play an important role in a wide range of clinical manifestations in leptospirosis patients. Mice with TLR4 defective are susceptible to infection.19 Although, there are increasing numbers of reports on Leptospira genes, there are a few studies on human genetics involving severe Leptospira infection. Single nucleotide polymorphisms in IL-4 and IL-4 receptor that had higher frequencies in leptospirosis patients were reported.53 However, it has been shown that IL-4 deficiency had no effect on resistance of BALB/C mice to Leptospira infection.51
Finally, the number of leptospires that infect each patient and the virulence of the infecting leptospires could play a role in different outcomes following Leptospira infection. However, currently, there is no report correlating leptospiral load and leptospirosis disease severity. In addition, disease outcomes in patients infected with pathogenic Leptospira with different degrees of virulence have not been compared.
Conflicts of interestThe authors declare no conflicts of interest.
Authors thank Rachadapiseksompoch research fund, Faculty of Medicine, Chulalongkorn University and the Higher Education Research Promotion and National Research University Project of Thailand (HR1155A), Office of the Higher Education Commission for the support of leptospirosis research.