Malaria is endemic in the North of Brazil. However, Hyperreactive Malarious Splenomegaly (HMS) has been rarely described. Splenomegaly in HIV/Aids infection has a large differential diagnosis, but malaria is a cause of gross splenomegaly, regardless of the HIV status. In this paper, we report the case of a 50-year-old man, HIV positive, with massive splenomegaly and multiple malaria infections in the past. He fulfilled the criteria for HMS, received a short course of anti-malarial treatment and weekly quimioprofilatic Chloroquine. In 9 months, he had great clinical and laboratorial improvement confirming the HMS, a rare diagnosis in Brazil.
Hyperreactive malarious splenomegaly (HMS), known as Tropical Splenomegaly Syndrome,1 despite being common in malaria-endemic regions and in travelers from non-endemic areas,2 has low prevalence in Brazil.3 The HMS is presented as an exaggerated immune response to infection by Plasmodium, resulting in nonspecific, hyper-immunoglobulin M (IgM) polyclonal immunoglobulin production by B lymphocytes, and the formation of immune complexes that are deposited in the spleen. The criteria for the diagnosis of HMS are: splenomegaly (>10cm below the left costal margin), increased IgM that exceeds twice the reference value, high titers of antibodies specific against Plasmodium sp. and clinical-laboratory improvement after treatment with anti-malarial drugs.4,5 The association with HIV infection is poorly documented, although HIV and malaria are endemic in many tropical regions.
Case reportA 50-year-old man was admitted to the infectious diseases sector of UHL (University Hospital of Londrina, Brazil) in February 2012. On admission, he complained of progressive weight loss, asthenia, and abdominal discomfort for two months, associated with fever for three days.
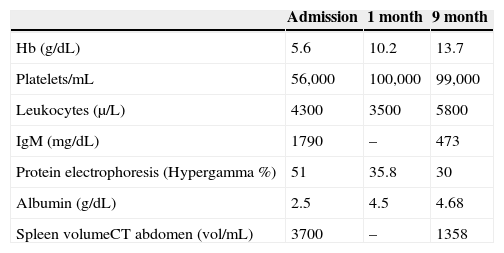
Physical examination showed discrete hepatomegaly and significant splenomegaly, about 20cm below the left costal margin. He reported having lived in the cities of Londrina/PR and Itaituba/PA, where he worked in mining for about seven months a year. He had a previous history of 20 episodes of malaria, and had been diagnosed with HIV in the past two years, with irregular use of antiretroviral therapy. Laboratory tests on admission showed: Hb=5.6g/dL, platelets=61,000/mm3; leukocytes=4300/mm3 (67% neutrophils), protein electrophoresis with hypergammaglobulinemia=51%; albumin=2.5g/dL; IgM=1790mg/dL (NR: 57-212), CD4=115/mm3 and viral load=2352copies/mL. Antibody (indirect immunofluorescence) anti-plasmodium vivax was 1/1280 and anti-plasmodium falciparum=1/320. The direct method for Plasmodium sp. thick smear was negative, as well as polymerase chain reaction (PCR). On admission, the first diagnostic hypothesis was sepsis after urinary tract infection. Once E. coli bacteria were isolated from blood and urine, the treatment with Ciprofloxacin had good response, but the urinary infection could not explain the splenomegaly.
Mieloculture was performed and fungi and Leishmania in two samples were searched, both turned out negative, ruling out histoplasmosis and visceral leishmaniasis. Inoculation in mice bone marrow blood resulted negative for Leishmania. Parasitical exams were negative in feces.
On admission, serology for toxoplasmosis and cytomegalovirus had positive IgM for both microorganisms, which ultimately proved to be false-positive. Likewise, anti-HCV was positive at low titers, with negative PCR for HCV.
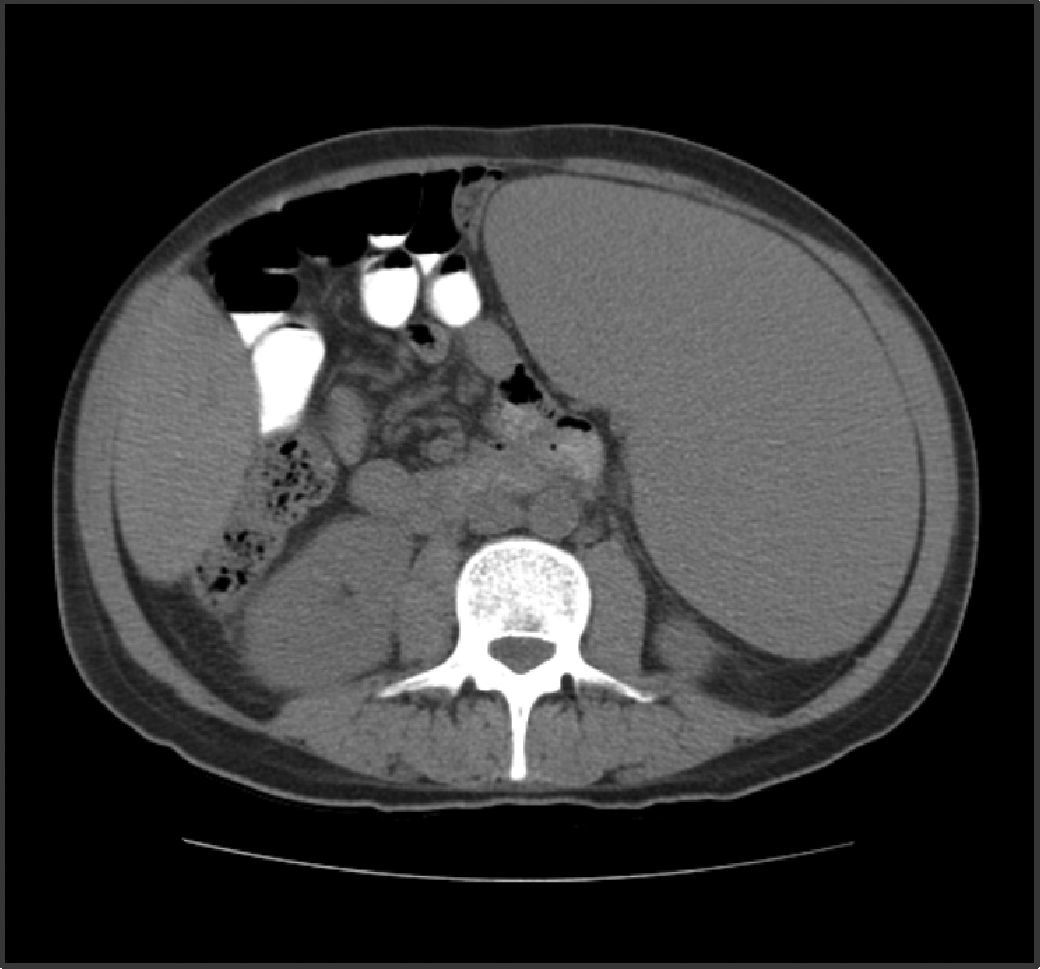
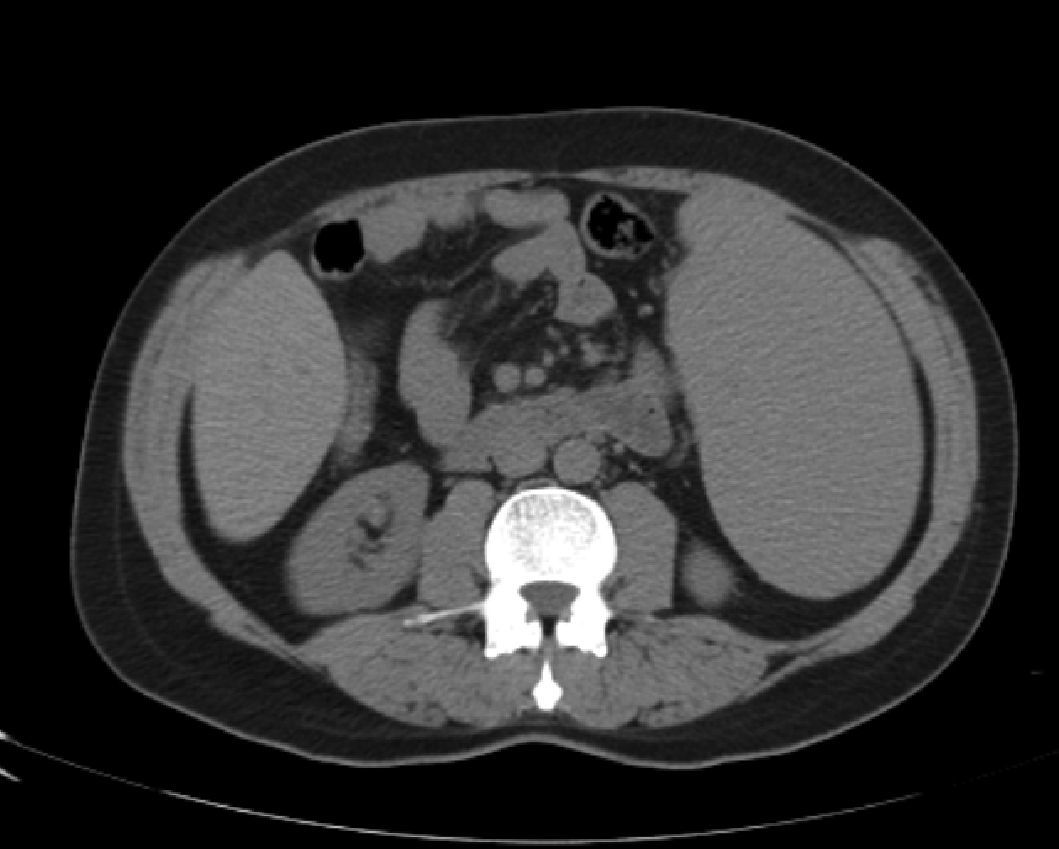
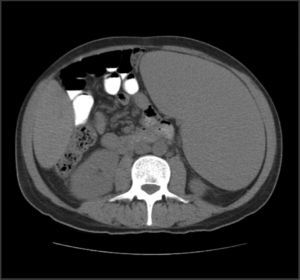
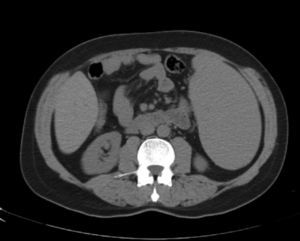
Abdominal tomography showed a 3700mL spleen at admission and 9 months after initiation of treatment, it reduced to 1358mL (64% reduction), demonstrating homogeneous parenchymal liver and spleen without portal hypertension (Figs. 1 and 2).
Having defined the diagnosis of HMS the first cycle with primaquine and chloroquine was carried out for 7 days, followed by chloroquine 300mg/week. Follow-up exams in the first and ninth month are shown in Table 1.
Discussion and conclusionThe development of HMS is associated with two main factors: repeated exposure to Plasmodium species and genetic predisposition. Countries with high endemism for malaria have prevalence of HMS ranging from 1 to 2% in Nigeria and 80% in certain tribes of New Papua Guinea.2 In Brazil, the prevalence is low in the Amazon region.3 Alecrim6 examined a population of 890 people living in a hyper-endemic area of Manaus, and found only 10 people with a persistent spleen enlargement. Of these, four met the criteria for Tropical Splenomegaly, as it was known at the time, three of them were brothers, underscoring the importance of genetic predisposition in tribal HMS.
The main genetic factors related to high population prevalence of HMS were HLA-DR2 and high levels of HLA heterozygous,4,7 in addition to the high frequency of the haplotype IGHG3.8
It is believed that patients with HMS have a hyper production of polyclonal IgM,1 which is linked to suppressor T lymphocytes (cytotoxic phenotype), causing the elimination of the parasite, as well as generating immune complexes that are deposited in the spleen, thus resulting in splenomegaly.
In this case, the overproduction of nonspecific polyclonal IgM explains the false positive results for both cytomegalovirus and toxoplasmosis, in addition to high titers of IgM (one of the diagnostic criteria).
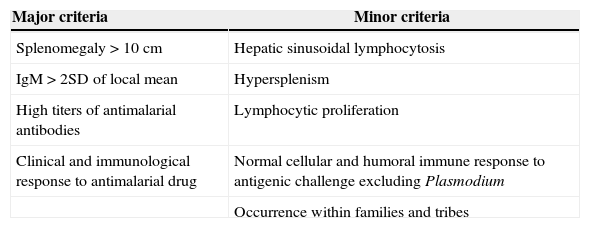
The diagnosis of tropical splenomegaly syndrome requires exclusion of other causes of massive splenomegaly. However, definite diagnosis depends on specific criteria, such as the Fakunle4,5,9 criteria shown in Table 2. These criteria are not different for HIV patients.10 In the case herein reported, the patient met all major criteria in addition to hypersplenism as a minor criterion, thus confirming the diagnosis.
Diagnostic feature of hyperreactive malarious splenomegaly (HMS) – Fakunle criteria.
| Major criteria | Minor criteria |
|---|---|
| Splenomegaly>10cm | Hepatic sinusoidal lymphocytosis |
| IgM>2SD of local mean | Hypersplenism |
| High titers of antimalarial antibodies | Lymphocytic proliferation |
| Clinical and immunological response to antimalarial drug | Normal cellular and humoral immune response to antigenic challenge excluding Plasmodium |
| Occurrence within families and tribes |
Although HIV/AIDS and malaria coexist with high prevalence in many countries of sub-Saharan Africa, few cases have been published linking the two diseases.10,11 In individuals with AIDS there is an inversion in the CD4/CD8 ratio due to decrease in number and function of CD4T lymphocytes, while in HMS there is an increase in this ratio due to decreased number of CD8T lymphocytes. One hypothesis is that this difference may prevent patients with HIV from manifesting HMS after repeated Malaria infections, explaining therefore the low number of cases associating the two diseases. However, further studies should be carried out to better identify the immunological relationship between them.
HMS treatment consists of a short cycle of anti-malarial drug, and the use of chemoprophylaxis (chloroquine in this case) for a period of 9–26 months in patients who remain exposed to malaria, to prevent new infections and to have the described immunomodulatory effect of chloroquine.12
Therefore, we must be alert to cases of massive splenomegaly in patients living in endemic areas of malaria in Brazil, or those who migrated from these regions. Furthermore, HMS needs to be considered as a differential diagnosis in patients with splenomegaly and AIDS.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank André Machado Siqueira from Universidade Estadual do Amazonas (Amazonas State University) for the suport in malaria PCR.