Carbapenem-resistant Pseudomonas aeruginosa is an important nosocomial pathogen associated with high mortality rates and can persist in moist environments in hospitals. Several outbreaks have been associated with environmental contamination, contaminated medical equipment, and healthcare cross-transmission. The production of acquired metallo-β-lactamase SPM-1 has been frequently associated to these infections.
Here, we report an outbreak of carbapenem-resistant P. aeruginosa encoding the blaSPM-1 MβL gene affecting nine patients under cancer treatment admitted at the Institute of Pediatric Oncology from November 2011 to May 2012. Strains were isolated from blood (n=7) and urine (n=2) and identified by BD Phoenix™ Automated System (BD Biosciences, Sparks Md). DNA was extracted using the QIAamp DNA mini-kit (QIAGEN, CA) and tested for MβL genes by Multiplex real-time PCR (Rotor-gene-Q, Qiagen, CA)1 followed by DNA sequencing (ABI sequencer, Applied Biosystems, USA). Clonal relatedness was evaluated by pulsed field gel electrophoresis (PFGE) using the SpeI restriction endonuclease (New England BioLabs, USA). The band patterns were analyzed using the Bionumerics Software 7.6 (Applied Maths, Belgium).
All nine P. aeruginosa isolates were resistant to all beta-lactams, fluoroquinolones, and aminoglycosides but susceptible to colistin. The samples were positive for the blaSPM-1 and negative for other MβL genes. PFGE demonstrated three different clusters, one of them was highly related to the endemic clone SPM-1 previously reported in the institution.2 This fact reinforces that a genomic variety recently observed among various SPM-1-producing P. aeruginosa isolates is result of the accumulation of mutations along the time and the endemic clone was now adapted to spread in-hospital ambient.3
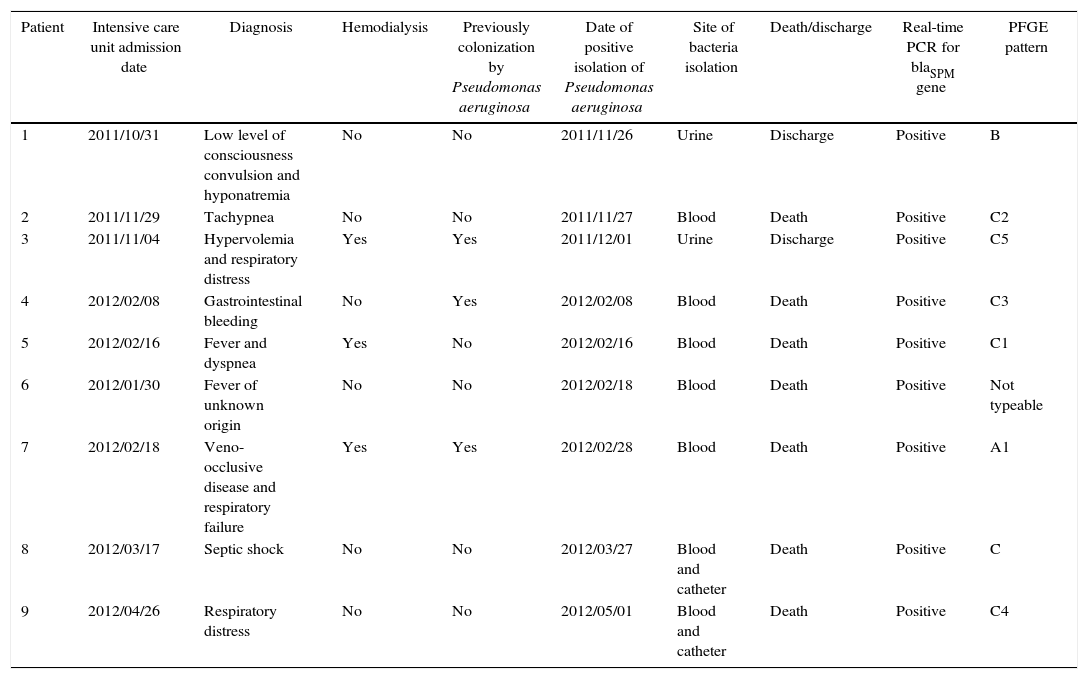
Three patients were previously colonized by P. aeruginosa and one had a contaminated prosthesis before the infection episode. Out of the nine patients, those who had the strain isolated from blood died (Table 1). All affected patients were critically ill and neutropenic. The majority of them presented septic shock and acute respiratory syndrome. Thus, we think these factors, combined with the lack of therapeutic options for these infections, could explain the observed high mortality rate.
Clinical data of infection episodes caused by SPM-1 producing Pseudomonas aeruginosa.
| Patient | Intensive care unit admission date | Diagnosis | Hemodialysis | Previously colonization by Pseudomonas aeruginosa | Date of positive isolation of Pseudomonas aeruginosa | Site of bacteria isolation | Death/discharge | Real-time PCR for blaSPM gene | PFGE pattern |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2011/10/31 | Low level of consciousness convulsion and hyponatremia | No | No | 2011/11/26 | Urine | Discharge | Positive | B |
| 2 | 2011/11/29 | Tachypnea | No | No | 2011/11/27 | Blood | Death | Positive | C2 |
| 3 | 2011/11/04 | Hypervolemia and respiratory distress | Yes | Yes | 2011/12/01 | Urine | Discharge | Positive | C5 |
| 4 | 2012/02/08 | Gastrointestinal bleeding | No | Yes | 2012/02/08 | Blood | Death | Positive | C3 |
| 5 | 2012/02/16 | Fever and dyspnea | Yes | No | 2012/02/16 | Blood | Death | Positive | C1 |
| 6 | 2012/01/30 | Fever of unknown origin | No | No | 2012/02/18 | Blood | Death | Positive | Not typeable |
| 7 | 2012/02/18 | Veno-occlusive disease and respiratory failure | Yes | Yes | 2012/02/28 | Blood | Death | Positive | A1 |
| 8 | 2012/03/17 | Septic shock | No | No | 2012/03/27 | Blood and catheter | Death | Positive | C |
| 9 | 2012/04/26 | Respiratory distress | No | No | 2012/05/01 | Blood and catheter | Death | Positive | C4 |
Although no common source could be identified, and given the limited therapeutic options for these infections, there is an urgent need for a better adherence of infection control practices in addition to investigate SPM-producing strains in routine diagnostic bacteriorology to prevent further dissemination of this challenging pathogen.
Conflicts of interestThe authors declare no conflicts of interest.