Klebsiella pneumoniae is widely recognized as a significant threat to human health, probably associated to the emergence of hypervirulent and pan-resistant strains associated with hospital infections.1Klebsiella quasipneumoniae is a novel, recently described, bacterial species which can be as virulent as other K. pneumoniae strains, causing invasive infections and resulting in high mortality rates.2 In this report, we report the first draft genome of a clinical ESBL-producing, colistin-resistant, highly virulent and hypermucoviscous K. quasipneumoniae subsp. similipneumoniae isolate in Brazil.
The KP121 strain was isolated from the rectal mucosa of a patient in a teaching hospital in Southeastern Brazil, on July 28, 2015. It was formerly characterized as K. pneumoniae at the hospital by the Vitek-2® system (bioMérieux, Brazil). Antimicrobial susceptibility tests were performed by Microflex TM matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Bremem, Germany), remaining sensitive to ciprofloxacin and tigecycline. Colistin susceptibility was confirmed by the broth microdilution method according to EUCAST guidelines, and >2μg/mL was considered resistant to colistin. It is important to note that, 13 days prior to sample isolation, the patient was colonized at the same site by a KPC-producing K. pneumoniae (positive blaKPC gene), both from different clones assessed by PFGE.3 At the time of KP121 strain isolation, the patient was on ceftriaxone, cefepime, and vancomycin due to other hospital infections, and was discharged 14 days after.
Total genomic DNA of the strain was subjected to whole genome sequencing (WGS) using Illumina NextSeq 500 sequencer (Illumina, San Diego, CA). Sequence reads were de novo assembled using the Velvelt pipeline (version 5.0.1) and Geneious (version R9). This assembly was submitted for annotation using the NCBI Prokaryotic Genome Annotation Pipeline, and pairwise alignment was performed by BLASTn homology searches (http://blast.ncbi.nlm.nih.gov). Multilocus sequence typing (MLST) (https://cge.cbs.dtu.dk//services/MLST/), Resfinder (https://cge.cbs.dtu.dk//services/MLST/) and PlasmidFinder (https://cge.cbs.dtu.dk//services/PlasmidFinder/) were used to determine the sequence type, antibiotic resistance genes, and plasmid types present in the isolate. The BLASTn tool and the pairwise alignment of Geneious also allowed comparisons with the genomic DNA of the NTUH-K2044 strain. Were examined chromosomal mutations in genes encoding PhoP/Q, PmrA/B and CrrA/B two-component systems and mutations or insertions in mgrB.
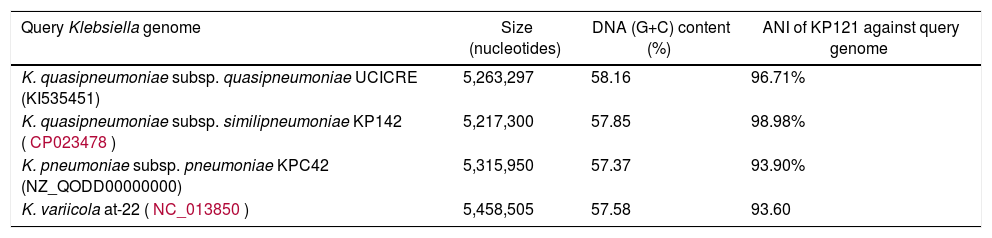
Analyses revealed that the strain belonged to the sequencing type (ST) 1484. KP 121 was identified as K. quasipneumoniae subsp. similipneumoniae based on two criteria: (i) a high pairwise average nucleotide identity (ANI) of 98.98% with the reference strain KP142 (CP023478.1); and (ii) presence of the blaOKP-B-2 gene (Table 1). The strain was placed in the KPIIb phylogroup which further supported subspecies classification. This strain presented the wzi sequence (wzi allele: 251) assigned to the (K-loci)-type KL16 (http://bigsdb.web.pasteur.fr/klebsiella).
Average nucleotide identity of KP121 genome against Klebsiella genomes.
| Query Klebsiella genome | Size (nucleotides) | DNA (G+C) content (%) | ANI of KP121 against query genome |
|---|---|---|---|
| K. quasipneumoniae subsp. quasipneumoniae UCICRE (KI535451) | 5,263,297 | 58.16 | 96.71% |
| K. quasipneumoniae subsp. similipneumoniae KP142 (CP023478) | 5,217,300 | 57.85 | 98.98% |
| K. pneumoniae subsp. pneumoniae KPC42 (NZ_QODD00000000) | 5,315,950 | 57.37 | 93.90% |
| K. variicola at-22 (NC_013850) | 5,458,505 | 57.58 | 93.60 |
Although K. pneumoniae is the most clinically relevant species within the genus,2 the emergence and description of a closely related highly virulent subspecies, hypermucoviscous and with extensive drug-resistant characteristics is significant, as it may be another important resistance and virulence gene reservoir. In epidemiological terms, plasmids from the incompatibility group IncHI2 were found which, according to the literature, are associated with multidrug-resistant (MDR) isolates.4
Although it belongs to the capsular type 16, the hypermucoviscosity characteristic of this strain was confirmed by the phenotypic test according to Catalán-Nájera et al.,5 which evidenced ˃5mm strings. In addition, the strain presented virulence genes encoding the aerobactin (iutA), type 1 fimbriae (fimABCDEFGHIK), type 3 fimbriae (mrkABCEFHJ), Escherichia coli common pilus (ecpA), outer core lipopolysaccharide (wabG), enterobactin (entB), urease synthesis associated genes (ureADE), allABCDRS, KfuABC, ugE and traT. In addition, this strain also produces a moderate level of biofilm.3
Previously published studies have assessed healthcare-associated infections (HAI) epidemiology caused by MDR microorganisms, indicating that these infections are highly prevalent and difficult to control.3 The description of a non-common, highly resistant strain makes this issue even more troubling. The strain described here is colistin-resistant, but none of the classical mutations responsible for this phenomenon was detected. However, other mutations not yet described in the literature were identified (S363I in pmrB, L424P in phoQ, Q99R; Q239H; Q287; L295K in crrB). Although we did not do experiments to confirm the relationship of these mutations with resistance to colistin, this may be related to this phenotype. Another strain of K. quasipneumoniae subsp. similipneumoniae was recently described in Brazil, which although susceptible to colistin presented the KPC gene, reinforcing the idea that strains of this lineage are an important reservoir of resistance genes.6 Unfortunately, the increased use of polymyxins has led to reports of colistin-resistant isolates worldwide, particularly in Brazil.3 Although this strain does not have any carbapenemase gene, it is resistant to carbapenems, as well as to aminoglycosides (mediated by aph(3′)-Ia, ant(2″)-Ia and aadA2), fluoroquinolones (mediated by qnrA1, oqxA and oqxB) and fosfomycin (mediated by fosA). In addition and more seriously, this strain presents the blaCTXM-9 gene, conferring an ESBL phenotype.
To the best of our knowledge, this is the first report of a highly virulent and hypermucoviscous colistin-resistant K. quasipneumoniae, which may represent a new challenge for clinical management and public health concern in Brazil. The description of this strain sets up a dark storm in hospital settings, due to the real possibility of a new resistance and virulence genes reservoir, which represents a challenge for the development of more effective surveillance and prevention programs.
Nucleotide sequence accession numberThe sequence of the KP121 strain was deposited in GenBank with accession number QXXP00000000.
Ethical approvalNot required.
FundingThis work was supported by the Brazilian Funding Agency FAPEMIG (Fundação de Amparo à Pesquisa de Minas Gerais) and Brazilian Funding Agency CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank members of Laboratory of Bacterial Resistance and Therapeutic Alternatives, Department of Microbiology – ICBII/USP, especially Dr Nilton Licopan and members of Laboratory of Bacteriology in Fish, especially Dr Ulisses de Pádua Pereira, for assisting in the analysis of sequencing.