Fusarium spp are ubiquitous fungi recognized as opportunistic agents of human infections, and can produce severe infections in burn patients. The literature on Fusarium spp infections in pediatric burn patients is scarce.
ObjectivesTo describe the clinical and epidemiological features as well as outcome of Fusarium spp infections in pediatric burn patients.
Patients and methodsRetrospective, descriptive study of Fusarium spp infections in a specialized intensive care burn unit.
ResultsIn 15 patients Fusarium spp infections were diagnosed. Median age was 48 months. Direct fire injury was observed in ten patients. The median affected burn surface area was 45%. Twelve patients had a full thickness burn. Fourteen patients had a Garces Index ≥3. Fungal infection developed at a median of 11 days after burn injury. Fungi were isolated from burn wound in 14 patients and from the bone in one patient.
Amphotericin B was the drug of choice for treatment followed by voriconazole. Median time of treatment completion was 23 days. One patient (7%) died of fungal infection-related causes.
ConclusionIn our series Fusarium spp was an uncommon pathogen in severely burnt patients. The burn wound was the most common site of infection and mortality was low.
Infection is the most common and severe cause of morbidity and mortality in burn patients.1 The longer survival of this population has increased the frequency of fungal infections. The most commonly isolated species is Candida spp and in recent times there has been an increase in filamentous fungi such as Aspergillus spp, Mucor spp, and Fusarium spp.2Fusarium spp – a filamentous fungus – is ubiquitous in the environment and can be found in water and in the soil.3 It mainly produces infections in individuals with immune disorders caused by tumors, use of cytostatics, steroids, presence of diabetes mellitus, and HIV infection among others. Burn patients are the most commonly affected immunocompetent hosts.1,4
Commonly isolated Fusarium spp are F. solani, F. oxysporum, F. proliferatum, F. verticilliodes, etc.5
The literature on Fusarium spp infections in pediatric patients with burns is limited. Most publications are case reports of disseminated Fusarium spp infections in cancer patients or fungal keratitis infections.6–8
The aim of this study was to describe the epidemiological, clinical, and microbiological features as well as outcome of infections due to Fusarium spp in pediatric burn patients.
Patients and methodsThis was a retrospective, descriptive and observational study of Fusarium spp infections in a specialized burn intensive care unit between January 2006 and March 2015.
Inclusion criteriaAll burn patients with clinical and microbiological evidence of fungal infection by Fusarium spp documented from representative samples, such as deep burn tissue, and/or blood, and/or other sterile sites with positive cultures were included.
DefinitionClinical evidence was defined by local signs of infection such as drainage of pus, redness or swelling at the margin of the burn, scar separation, localized necrosis, vesicular lesions, and/or poor adherence of the graft.
Microbiological evidence was defined by the isolation of Fusarium spp in deep tissue samples or sterile materials (blood, purulent materials, and/or urine).
Infection typesInfections were defined according to the American Burn Association9:
The Garces’ Index – an index of prediction of severity and mortality calculated as follows: 40−patient age×1 percentage burn (if type A), ×2 (if type AB), ×3 (if type B).
- •
0–60 points: Grade 1 (slight risk);
- •
61–90 points: Grade 2 (moderate risk);
- •
91–120 points: Grade 3 (severe risk);
- •
>121 points: Grade 4 (critical risk).
- •
Type A: superficial burn with erythema and hyperalgesia;
- •
Type AB: pink-white intermediate-color scar, and hypoalgesia;
- •
Type B or “full thickness”: deep, white or black scar and analgesia.
In addition to regular bacteriological study, all materials were processed for mycological study.
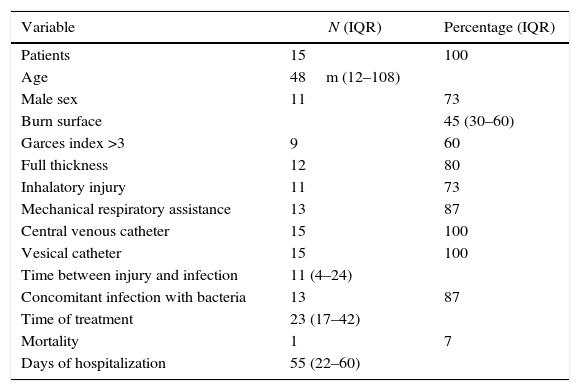
ResultsA total of 15 patients were infected by Fusarium spp during the study period. The mechanism of burn was direct fire in 10 patients (66%), hot liquids in four (27%), and electricity in one case (7%); 87% (n=13) were male. Median age was 48 months (IQR 12–108 months). The median body surface area burned was 45% (IQR 30–60%). Eighty percent of patients (n=12) had deep burns (type B); 60% (n=9) had a Garces’ index of 3, 33% (n=5) an index of 4, and the remaining 7% (n=1) an index of 2.
The median time from burn injury to onset of fungal infection was 11 days (IQR 4–24 days). All patients had central lines with a median dwell time of 20 days (IQR 12–30). Of all patients, 87% (n=13) required mechanical ventilation with a median of 16 days (IQR 10–30). The most common site of microbiological isolation was the burn wound in 15 patients (100%) and bone in one patient (7%). In one patient (7%) Fusarium spp was isolated from blood cultures. In 87% of cases (n=13) concomitant bacterial infections were found. Empirical antifungal treatment was started with amphotericin B deoxycholate in 14 patients (93%) and voriconazole in one patient (7%). All patients were switched to treatment with voriconazole after microbiological isolation of Fusarium spp. Median antifungal treatment was 23 days (IQR 17–42 days). No adverse events related to the use of antifungal agents were reported. The combination of medical treatment using amphotericin B, voriconazole, and surgical removal of infectious foci was effective in all cases. The median length of hospital stay was 55 days (IQR 22–60 days). Only one patient (7%) died, 14 days after admission due to septic shock related to Fusarium spp infection (Tables 1 and 2).
Patients characteristics.
| Variable | N (IQR) | Percentage (IQR) |
|---|---|---|
| Patients | 15 | 100 |
| Age | 48m (12–108) | |
| Male sex | 11 | 73 |
| Burn surface | 45 (30–60) | |
| Garces index >3 | 9 | 60 |
| Full thickness | 12 | 80 |
| Inhalatory injury | 11 | 73 |
| Mechanical respiratory assistance | 13 | 87 |
| Central venous catheter | 15 | 100 |
| Vesical catheter | 15 | 100 |
| Time between injury and infection | 11 (4–24) | |
| Concomitant infection with bacteria | 13 | 87 |
| Time of treatment | 23 (17–42) | |
| Mortality | 1 | 7 |
| Days of hospitalization | 55 (22–60) |
Infections remain the leading cause of mortality in burn patients.1 The use of topical and systemic antimicrobials along with infection-control measures has reduced the incidence of bacterial infections1; however, fungal infections have remained stable showing an incidence between 6 and 40% in different series.1,5,6
In a previous study conducted at our center we reported on 128 infections in 84 patients of which 53 cases (20%) had a documented fungal etiology.4 Some risk factors for developing fungal infections in burn patients are extremes of age, large body surface burned (≥40%), inhalation syndrome, requirement for mechanical ventilation, use of broad spectrum antibiotics, prolonged use of central venous and bladder catheters, presence of diabetes mellitus, and parenteral nutrition.2,3,10,11
Progression from fungal colonization to infection of the wound is directly related to the body surface area burned. Therefore, close monitoring of patients with large surfaces burned (≥40%) is recommended to establish early therapeutic measures.12
Different studies in the literature have reported a higher incidence of fungal infections in more severe burns.1,3,4,6 In this series, the median body surface area burned was 45%, reflecting the severity of the study sample at high risk for the occurrence of fungal infections in line with published literature.6,10
Candida spp is the most frequently involved species in fungal infections in burns. In recent times the incidence of filamentous fungi such as Aspergillus spp, and to a lesser extent Mucor spp and Fusarium spp, has increased.2 In a previous study on fungal infections in our center the most frequently isolated fungus was Candida albicans (3.4%).13 In this study fungal infections occurred from the second week of hospitalization (median 11 days), coinciding with several other reports.2,3,11
Fusarium can be isolated by tissue biopsies that may show the presence of filamentous fungi in and around vessels of the dermis, thrombosis, and tissue necrosis. Blood cultures may be positive in 50–70% of cases in immunocompromised host infections.14,15
The most common site of infection is the burn wound.1,3,4,14 In this study 100% of patients had infection of the burn wound and only one (7%) had bone involvement. Additionally, fungemia was detected in only one patient (7%).
Early treatment of localized forms is essential to prevent the spread of Fusarium spp. Mortality rates have been reduced with early debridement of injuries combined with antifungal treatment.15–17
In our burn unit, amphotericin B deoxycholate or lipid formulations are used as empirical treatment against filamentous fungi. When Fusarium spp is isolated voriconazole is the drug of choice. The total duration of treatment varies according to the extension of the infection. Some studies reported a treatment duration of at least two weeks after the last fungal isolation coinciding with the management approach in our burn unit.2,11,18 Mortality due to fungal infections varies between 75 and 90% and is associated with a burned surface area between 40% and 60%.1,10,19 Some studies found no difference in mortality rates according to the fungal agent involved. In contrast, the study by Schaal et al.20 found that patients with Aspergillus spp infections had a 30% mortality whereas none of those infected with Fusarium spp died, in line with the low mortality rate found in our study.
Nir Paz et al.3 reported an 11% mortality rate in Fusarium spp infections in different inmunocompromised patients with a higher risk in patients with burns.
In our series, one patient died of septic shock due to infection by Fusarium spp, representing a mortality rate of 7% in the study period, which is lower than that found in the literature.11–20
ConclusionsFusarium spp was a rarely isolated pathogen in burned patients in our unit. The burn wound was the most frequent site of isolation. Mortality was low.
Conflicts of interestThe authors declare no conflicts of interest.