Fluconazole is extensively used for the treatment of candidiasis and cryptococcosis. Among other factors, successful treatment is related to appropriate fluconazole levels in blood and cerebrospinal fluid. In the present study, fluconazole levels were determined in 15 patients, 14 of whom had AIDS and 13 had neurocryptococcosis. The only selection criterion was treatment with fluconazole, which was performed with a generic or similar form of the drug. Fluconazole level was determined by high performance liquid chromatography and the susceptibility profile of Cryptococcus spp. isolated from the patients was assessed by broth microdilution. Blood and cerebrospinal fluid fluconazole levels were found to be related to the fluconazole daily dose, and exceeded the minimum inhibitory concentration of this antifungal for the Cryptococcus spp. isolates. A good correlation was observed between serum and cerebrospinal fluid drug concentration. In conclusion, treatment with non-original fluconazole under usual medical practice conditions results in appropriate blood and cerebrospinal fluid levels of the drug for inhibiting Cryptococcus spp. susceptible to this antifungal drug. The relatively common failures of neurocryptococcosis treatment appear not to be due to insufficient fluconazole levels in the cerebrospinal fluid, especially with the use of daily doses of 400–800mg.
Fluconazole is extensively used as a systemic antifungal agent because of its favorable pharmacological characteristics such as low toxicity, good bioavailability and appropriate levels in blood and in infected tissues, including the central nervous system (CNS). In addition, fluconazole acts against various agents that induce endemic and opportunistic mycoses, with emphasis on its clinical use for the treatment of Candida spp. and Cryptococcus spp. infections.1,2
Together with amphotericin B and 5-flucytosine, fluconazole is a component of the therapeutic regimen currently recommended for the control of neurocryptococcosis, particularly during treatment consolidation and maintenance phases.3 However, some cases are refractory to antifungal therapy and early or late deaths may occur in more than 30% of all patients.4,5 Poor response to treatment has been related to more extensive dissemination of the infection and to host factors.6 However, treatment failure may also be due to insufficient antifungal dosage and tissue level or to the lack of Cryptococcus spp. susceptibility to fluconazole.7
Early studies have shown that fluconazole reaches sufficient levels in cerebrospinal fluid (CSF) for the treatment of fungal infections in the CNS.8 In contrast to controlled studies, in current clinical practice the patients receive fluconazole of various origins and the clinical conditions may differ from those standardized in studies with the original drug. Scarce data about generic antifungals motivated a comparison between innovator fluconazole and similar drugs in an experimental model.9 For the same reason, in this study fluconazole levels were reevaluated in blood and CSF of patients under treatment for cryptococcosis and other fungal infections.
Materials and methodsFifteen adult patients were included at random in the present study while receiving fluconazole treatment between September 2012 and May 2014 at the University Hospital of the Ribeirão Preto Medical School, University of São Paulo, Brazil. All but one of these patients were HIV-infected and fluconazole was being used to treat neurocryptococcosis (n=13), histoplasmosis (n=1) or candidiasis (n=1). Neurocryptococcosis cases were preferably included because of periodic CSF sampling and the use of different fluconazole dosages during treatment, permitting the comparison of the levels of this drug according to daily dose received. Cryptococcus spp. was isolated in Sabouraud agar to which the CSF was added and identified by standard medical mycology methods. This identification was confirmed by Vitek 2 automated system (Biomeriéux, France). Histoplasmosis was diagnosed by histopathogical examination and a positive serology for Histoplasma capsulatum antibodies. Oral candidiasis was diagnosed by oral examination of an AIDS patient. The following brands of fluconazole were administered to the patients: generic Fluconazol (Sanobiol, Brazil) for intravenous infusion; capsules for oral use – Fluxilase®, (Laboris, Brazil) or Flucazol® (Cristália, Brazil). The patients received fluconazole doses of 200–800mg/day intravenously (n=4), orally (n=5) or by intravenous route followed by oral route (n=6). Nine patients were included in the study more than once, five of them for receiving a different daily dose and/or for using another route of administration. Amphotericin B and antiretrovirals were used simultaneously to fluconazole in 8/13 and 10/13 patients with cryptococcosis, respectively. Among antiretroviral drugs, all 10 patients used lamivudine, and other accompanying drugs of the regimen were combinations of tenofovir or zidovudine with efavirenz or lopinavir/ritonavir.
Blood and CSF samples were collected between 24h and 80 days after the beginning fluconazole treatment. CSF was sampled only when requested by medical staff for laboratory control of cryptococcosis treatment. Drug levels were measured in blood samples collected one or two hours after administering a fluconazole dose. CSF was collected by lumbar puncture three to four hours after ingestion or infusion of a fluconazole dose. Fluconazole levels were measured in two to seven blood samples per patient for a total of 53 samples, and in one to four CSF samples for a total of 22 samples. The correlation between serum and CSF concentrations of the drug was determined in 10 patients from whom 15 sample pairs were collected on the same day.
Fluconazole levels were measured by high performance liquid chromatography (HPLC).10 Fluconazole was extracted from serum and CSF by liquid–liquid extraction using ethyl acetate as an organic solvent. HPLC was carried out using a Shimadzu chromatograph (Shimadzu Corporation, Kyoto, Japan), with a Supelco Analytical Ascentis 4.6mm×25cm C18® column with 5μm particles, an Ascentis C18 Supelguard® guard column (4mm×2cm, with 5μm particles) and a mobile phase consisting of 10mM sodium phosphate buffer, pH 5.7, and acetonitrile (75:25, v/v) (Merck Darmstad, Germany), at a flow rate of 1mL/min, with detection at 210nm. The HPLC method was validated for fluconazole (Sigma–Aldrich, USA) quantification in serum and CSF. In the range of 0.5–62.5μg/mL fluconazole concentration, the correlation coefficients were 1.0000 and 0.9998 for serum and CSF, respectively. The precision expressed as variation coefficient percentage ranged 0.5–8.1 for serum and 0.7–4.5 for CSF in intra-run and inter-run analysis. Cryptococcus spp. susceptibility to fluconazole was determined in isolates from 11 patients by broth microdilution method as proposed by the Clinical Laboratory Standards Institute.11
Fluconazole levels were analyzed according to neurocryptococcosis patients’ outcome after one year of diagnosis. Two groups of patients were compared: survivors – 9/13 cases vs. deaths plus one case of refractory cryptoccocosis – 4/13 cases. In the survival group the median age was 43 years (range: 21–63), 8/9 were men and 5/9 and 6/9 were also receiving amphotericin B and antiretrovirals, respectively. Five patients were in the consolidation phase of antifungal therapy and four of them had been previously treated with amphotericin B. In the death/refractory group the median age was 49 years (range: 32–65), all were men and 3/4 and 2/4 were also receiving amphotericin B and antiretrovirals, respectively. One patient was in the consolidation phase of treatment and had received amphotericin B previously.
The Mann–Whitney test was used to compare blood and CSF fluconazole levels according to the daily dose of the drug received by the patients, with the level of significance set at p<0.05. The correlation between blood and CSF levels of the drug was determined using Excel® software (Microsoft Corporation). The study was approved by the Research Ethics Committee of the University Hospital, Ribeirão Preto Medical School (protocol no. 4096/2012) and all patients gave written informed consent to participate.
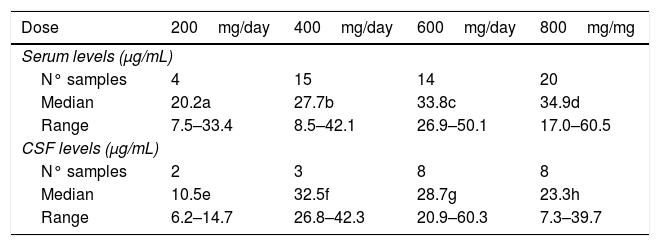
ResultsTable 1 shows that the median serum levels of fluconazole increased with increasing daily doses of the drug, reaching a significant difference between the daily dose of 600 or 800mg/d and the dose of 400mg/d. The lowest median serum level was observed at the dose of 200mg/d, although the small number of samples prevented reaching significance compared to the higher levels provided by daily doses between 400 and 800mg.
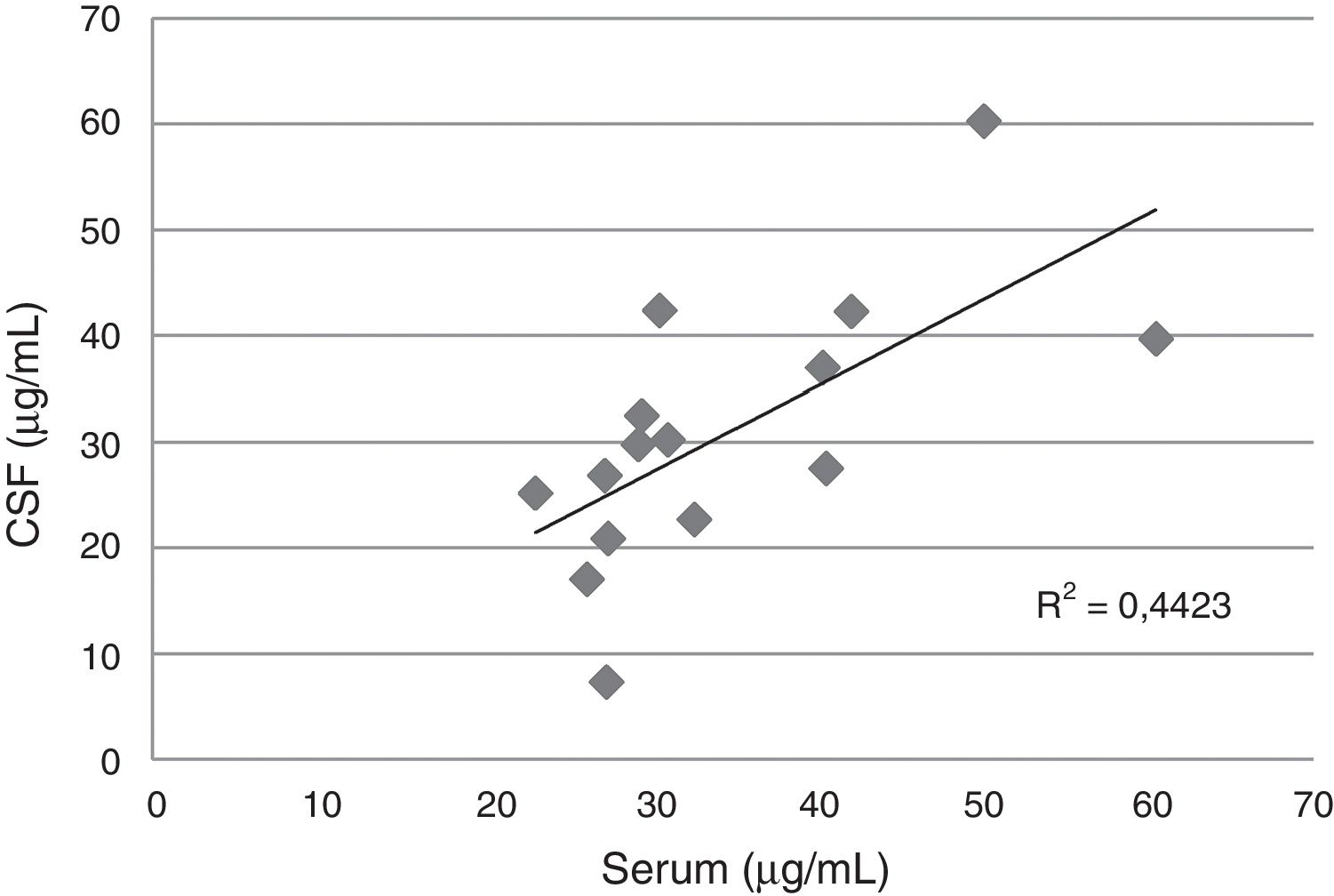
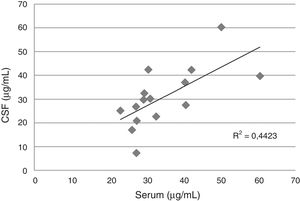
Serum and cerebrospinal fluid (CSF) fluconazole levels according to daily dose administered to 15 patients.
| Dose | 200mg/day | 400mg/day | 600mg/day | 800mg/mg |
|---|---|---|---|---|
| Serum levels (μg/mL) | ||||
| N° samples | 4 | 15 | 14 | 20 |
| Median | 20.2a | 27.7b | 33.8c | 34.9d |
| Range | 7.5–33.4 | 8.5–42.1 | 26.9–50.1 | 17.0–60.5 |
| CSF levels (μg/mL) | ||||
| N° samples | 2 | 3 | 8 | 8 |
| Median | 10.5e | 32.5f | 28.7g | 23.3h |
| Range | 6.2–14.7 | 26.8–42.3 | 20.9–60.3 | 7.3–39.7 |
Significant differences by the Mann Whitney test: b vs. c – p<0.05; b vs. d – p<0.01; e vs. f – p<0.05; e vs. g – p<0.05; e vs. h – p<0.05
In the CSF, the median levels of fluconazole were similar with daily doses between 400 and 800mg/d, but exceeded the levels obtained with 200mg/d. A good correlation between serum and CSF levels was detected in 15 blood-CSF pairs in patients receiving daily doses between 400 and 800mg (Fig. 1).
Cryptococcus isolated from the patients showed susceptibility to fluconazole, as demonstrated by a MIC between 0.125 and 2.0μg/mL, MIC 50=0.5μg/mL and MIC 90=2.0μg/mL.
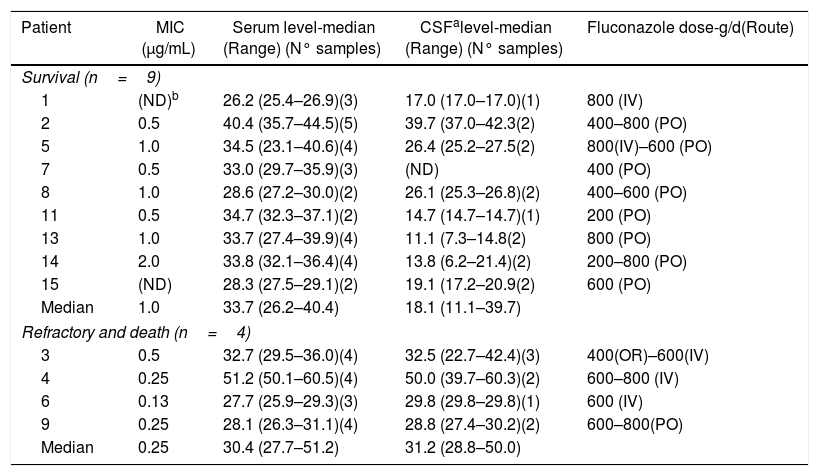
No differences were verified in MIC distribution and of serum fluconazole levels in patients with cryptococcosis who survived compared to those with a non-favorable outcome. CSF drug levels of the death/refractory group were higher than levels of the survival group (Table 2).
Serum and CSF fluconazole levels (μg/mL) in cryptococcal meningitis cases and minimum inhibitory concentration (MIC) of fluconazole for Cryptococcus spp. isolates according to outcome of the patients.
| Patient | MIC (μg/mL) | Serum level-median (Range) (N° samples) | CSFalevel-median (Range) (N° samples) | Fluconazole dose-g/d(Route) |
|---|---|---|---|---|
| Survival (n=9) | ||||
| 1 | (ND)b | 26.2 (25.4–26.9)(3) | 17.0 (17.0–17.0)(1) | 800 (IV) |
| 2 | 0.5 | 40.4 (35.7–44.5)(5) | 39.7 (37.0–42.3(2) | 400–800 (PO) |
| 5 | 1.0 | 34.5 (23.1–40.6)(4) | 26.4 (25.2–27.5(2) | 800(IV)–600 (PO) |
| 7 | 0.5 | 33.0 (29.7–35.9)(3) | (ND) | 400 (PO) |
| 8 | 1.0 | 28.6 (27.2–30.0)(2) | 26.1 (25.3–26.8)(2) | 400–600 (PO) |
| 11 | 0.5 | 34.7 (32.3–37.1)(2) | 14.7 (14.7–14.7)(1) | 200 (PO) |
| 13 | 1.0 | 33.7 (27.4–39.9)(4) | 11.1 (7.3–14.8(2) | 800 (PO) |
| 14 | 2.0 | 33.8 (32.1–36.4)(4) | 13.8 (6.2–21.4)(2) | 200–800 (PO) |
| 15 | (ND) | 28.3 (27.5–29.1)(2) | 19.1 (17.2–20.9(2) | 600 (PO) |
| Median | 1.0 | 33.7 (26.2–40.4) | 18.1 (11.1–39.7) | |
| Refractory and death (n=4) | ||||
| 3 | 0.5 | 32.7 (29.5–36.0)(4) | 32.5 (22.7–42.4)(3) | 400(OR)–600(IV) |
| 4 | 0.25 | 51.2 (50.1–60.5)(4) | 50.0 (39.7–60.3)(2) | 600–800 (IV) |
| 6 | 0.13 | 27.7 (25.9–29.3)(3) | 29.8 (29.8–29.8)(1) | 600 (IV) |
| 9 | 0.25 | 28.1 (26.3–31.1)(4) | 28.8 (27.4–30.2)(2) | 600–800(PO) |
| Median | 0.25 | 30.4 (27.7–51.2) | 31.2 (28.8–50.0) | |
The most relevant result of the present study was that fluconazole concentrations reached in blood and CSF are sufficient to inhibit Cryptococcus spp. isolated from the patients of this series. Fluconazole doses higher than 200mg/d led to higher levels in CSF, which were safer regarding their antifungal action in cases of meningeal cryptococcosis.
The pharmacokinetics of fluconazole is linear and dose-dependent, and higher daily doses of the drug have been shown to result in longer survival of patients with candidiasis.12 The better pharmacokinetics parameter associated with fluconazole efficacy is the area under the curve of fluconazole blood concentration (AUC) divided by MIC (AUC/MIC) which must be ≥100 for Candida species.13 AUC near 386/L/h can be obtained with 400mg/day of fluconazole and this dose is sufficient to control 99% of Candida spp. infections whose MIC is ≤2μg/mL.14 In respect to cryptococcosis, it is more complicated to define pharmacokinetic parameters for better outcomes because of several factors including a commonly meningeal infection and a non-rare cerebral involvement. An experimental study with four Cryptococcus isolates estimated fluconazole mean AUC/MIC=389 for a fungistatic effect.15 In a murine model of cryptococcosis, fluconazole cerebral levels were lower than blood levels which could be lower than the MIC for Cryptococcus spp.15,16 Fluconazole doses and respective blood and CSF levels have been associated with neurocryptococcosis cases outcome. A dose ≥1200mg/day was recommended if this triazole is employed in monotherapy.3
The serum levels of fluconazole measured in the present study are comparable to those reported in other studies using daily doses of 200–400mg13,17 or of 800mg.18,19 In patients with cryptococcosis treated with daily doses of 800 and 1000mg/day the mean levels of serum fluconazole were 37.0μg/mL and 42.5μg/mL,20 respectively. A mean trough serum concentration of 5.6 (range: 0.11–18.0) μg/mL was verified in hematologic patients using 200mg/day of this triazole.21 There was no significant difference between the levels obtained when the drug was administered by oral or intravenous route (data not shown).
The levels of fluconazole detected here in the CSF were close to those observed in other studies on patients with cryptococcosis and HIV co-infection, whose mean CSF levels of the drug reached 25.1μg/mL, 32.7μg/mL, and 36.3μg/mL with daily doses of 400mg, 800mg, and 1000mg, respectively.19,20 The levels of fluconazole in the CSF and in blood showed a good correlation, in agreement with the concept that this drug has similar concentrations in the two fluids.22 Blood and CSF fluconazole concentrations observed in this study are apparently not related to the bad outcome of some patients.
All Cryptococcus spp. isolates were susceptible to fluconazole based on the epidemiological cut-off estimated at 8μg/mL.23 Resistance or non-susceptibility of Cryptococcus spp. to fluconazole may be the reason for treatment failure in cryptococcosis, especially if only this antifungal agent is used for treatment.2 Refractory/relapsing cryptococcal infection has been associated with Cryptococcus neoformans not being susceptible to fluconazole.24 Fluconazole at the dose of 800mg/d or alternative treatment with voriconazole was necessary to maintain survival of patients infected with Cryptococcus spp. with a MIC above 8μg/mL for this drug.7 A slow or delayed recovery of patients with neurocryptococcosis should indicate a Cryptococcus spp. susceptibility test and the monitoring of fluconazole levels in the CSF.25,26
In this study it was not possible to estimate several pharmacokinetic parameters of fluconazole. However, it confirmed that levels of this triazolic were directly related to the daily dose received by patients, which must be carefully managed, particularly in neurocryptococcosis cases. Another limitation of the study refers to the higher percentage of patients already in consolidation phase of treatment in the group of survivors in comparison to the death/refractory cryptococcosis group, impairing the analysis of the fluconazole levels according to outcome. Interaction of fluconazole with other drugs prescribed to meningeal cryptococcosis cases may occur, but amphotericin B and antiretrovirals in particular do not modify fluconazole levels.27
In conclusion, fluconazole reached sufficient blood and CSF levels to act against most Cryptococcus spp. that cause meningitis in AIDS patients, even though it was administered under non-standardized clinical conditions and the fluconazole used was not the original brand used to evaluate pharmacokinetics in initial studies. Daily doses of 400mg or higher provided more appropriate CSF concentrations for treating Cryptococcus spp., particularly isolates with lower susceptibility to fluconazole.
Conflicts of interestThe authors declare no conflicts of interest.
LA Schiave received a scholarship from the São Paulo Research Foundation (FAPESP) related to a research project on the determination of fluconazole levels (grant 2010/51030-4). The Foundation of Support to Teaching, Research and Assistance of Hospital das Clínicas, Ribeirão Preto Medical School (FAEPA) provided financial support for this study. We thank John Carpenter, Ribeirão Preto, SP, Brazil, for the English revision.