Antiretroviral therapy (ART) has modified the outcome of patients with HIV infection, providing virological control and reducing mortality. However, there are several reasons as to why patients may discontinue their antiretroviral therapy, with adverse events being one of the main reasons reported in the literature. This is a case-control nested in a cohort of people living with HIV/AIDS, conducted to identify the incidence of ART modification due to adverse events and the associated factors, in two referral services in Recife, Brazil, between 2011 and 2014. Of the modifications occurred in the first year of ART, 25.7% were driven by adverse events. The median time elapsed between initiating ART and the first modification due to adverse events was 70.5 days (95% CI: 26-161 days). The main adverse events were dermatological, neuropsychiatric and gastrointestinal. Dermatological events were the earliest to appear after initiating ART. Efavirenz was the most prescribed and most modified drug during the study period. The group of participants who used zidovudine, lamivudine, and efavirenz had a 2-fold greater chance (adjusted OR: 2.16 95% CI: 1.28-3.65) of switching ART due to adverse events when compared to the group that used tenofovir with lamivudine and efavirenz.
Antiretroviral therapy (ART) in Brazil is provided for free by the government according guidelines of the Ministry of Health. From 2008 to 2012 the first-line antiretroviral regimen for the treatment of HIV infection in Brazil was mainly based in a combination of zidovudine, lamivudine plus efavirenz or zidovudine, lamivudine plus lopinavir/ritonavir.1 From 2013 to 2017 the preferred regimen was a combination of tenofovir, lamivudine plus efavirenz or nevirapine, while zidovudine was an alternative nucleoside reverse transcriptase inhibitor (NRTIs). Ritonavir-bosted protease inhibitors (PI/r), represented by lopinavir/ritonavir or atazanavir/ritonavir, were a second option for initiating treatment, prescribed when there was contraindication for the use of non-nucleoside reverse transcriptase inhibitors (NNRTIs).2
A number of factors may contribute to discontinuing or modifying the initial therapy, such as adverse events; clinical, immunological or virological failure; simplification of therapy; lack of patient compliance; or drug interaction.3
Some studies have demonstrated that the main factor for discontinuing first-line ART regimen is adverse events, reducing the durability of the initial regimen.4–6
Although drug-related adverse event is the major cause of ART modification, the associated factors are poorly understood, especially in developing countries.7,8 Understanding these factors is important for therapeutic suitability according to the characteristics of the patients, thereby improving tolerability to treatment.4
The aim of this study was to investigate factors associated with the modification of first-line antiretroviral therapy due to the occurrence of adverse events amongst people living with HIV/AIDS.
The importance of this study is the fact that it investigates the first-line antiretroviral regimens recommended by the Brazilian Ministry of Health, at a time when Brazil expanded antiretroviral coverage and began to recommend treatment for all people living with HIV, regardless of their CD4 count. The study describes the frequency of modifications of first-line ART due to adverse events in the first year of use, and the major adverse events, and also investigates associated factors.
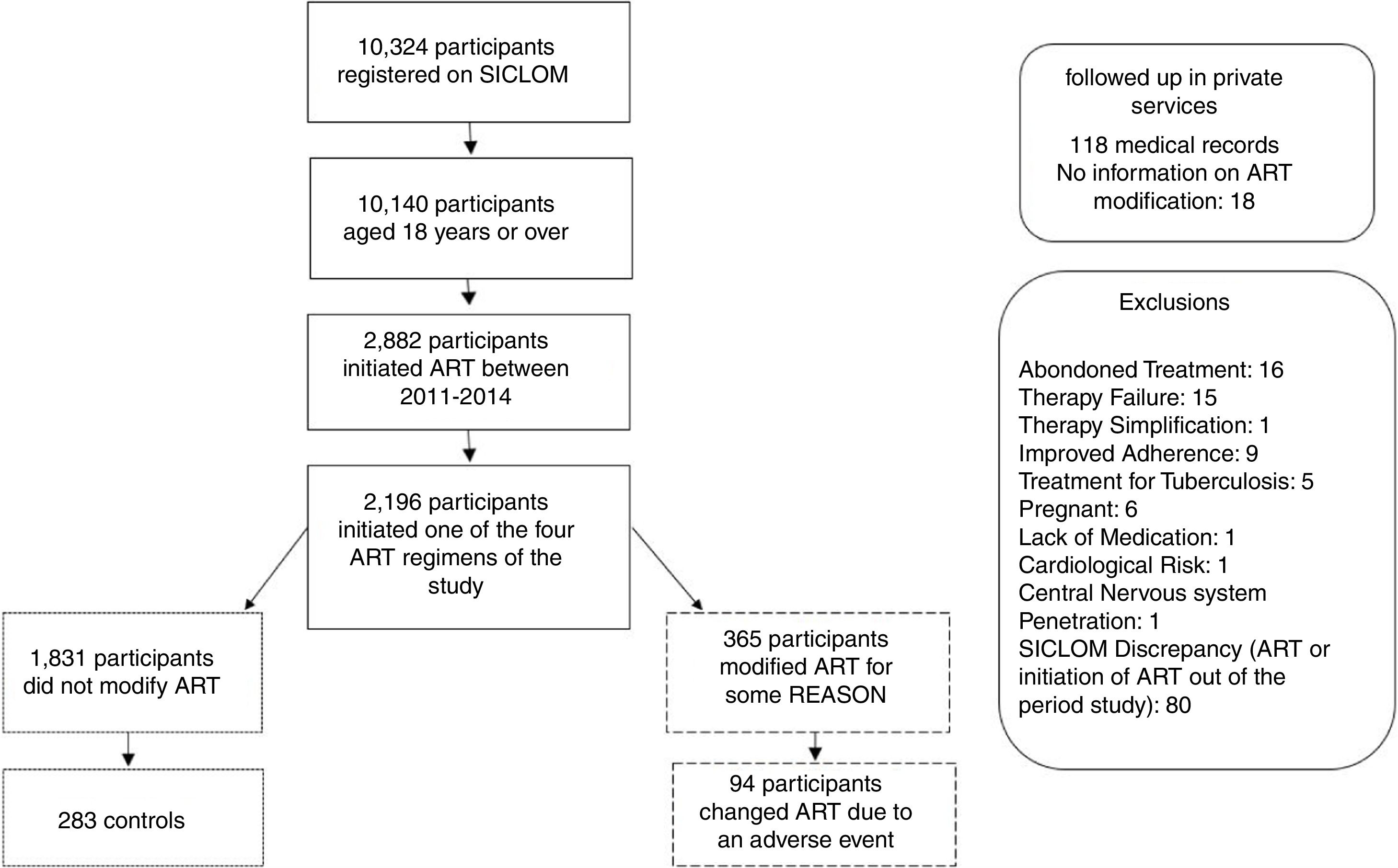
Material and methodsStudy design, population and data collectionThe study was a case-control study nested in a cohort of people living with HIV/AIDS, conducted at two referral services for HIV/AIDS in Recife-PE, Brazil. The study enrolled people aged ≥18 years, in the first year of ART, between 2011 and 2014, as ART information was available in the Logistic Control System for Antiretroviral Medication (known as SICLOM) from 2011 onwards. The study period comprises the modification in the first-line ART by the Ministry of Health in 2013.
Participants whose treatment had been modified during the first year of treatment due to adverse events were considered the case group. The maximum limit for ART modification was 15 months, since it would be possible for participants to present with mild adverse events and wait for up to three months to return for the medical reassessment consultation. In the control group, we included those whose therapeutic regimen was not modified in the first year of use. The control group was randomly selected, in the ratio of 1 case to 3 controls.
Data collection was initiated by seeking information from SICLOM about ART modifications. Next, the medical records of individuals who had their ART modified during the study period were reviewed to identify the reason for the change. We defined modification of ART as being the replacement of one or more drugs. Adverse event was defined as being a clinical or laboratory change attributed to the antiretroviral being used, recorded in medical records by the attending physician at the time of the modification.
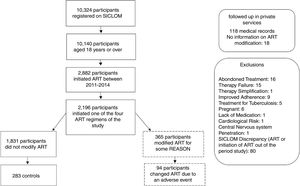
Patients whose first-line ART was modified for reasons other than an adverse event were excluded, as well as patients who abandoned ART in the first year of treatment, pregnant women, and those who initiated treatment with regimens other than those recommended by the Ministry of Health during the period 2011 to 2014. Those patients who only received ART in one of the two referral services of the study, but were followed up in private services, were considered as losses due to lack of medical records.
A database with information of those who did not have their therapy modified was created, from which the control group was randomly selected. Following, information was retrieved from medical records of cases and controls. The study sample consisted of 94 cases and 283 controls. Assuming this sample size and a significance level of 5%, the power of the study was estimated to be 70%, based on the study by Shet et al., that found a relative risk of 2.49 for occurring adverse events in people with CD4>250 cells/mm³, and data from the AIDS-PE cohort that found 65% of patients on initiation of antiretroviral therapy with CD4>200 cells/mm³.
This research was approved by the Ethics Committee HUOC/PROCAPE, in accordance with Resolution 466/12, with CAEE 56613816.3.0000.5192, 06/07/2016.
Study variablesThe dependent variable was modification in first-line antiretroviral therapy. The independent variables of the study were grouped into sociodemographic and life habits (age, sex, schooling, drug use and alcohol use); clinical (tuberculosis, neurotoxoplasmosis, pneumocystosis, oropharyngeal or esophageal candidiasis); therapeutic (first-line antiretroviral regimen, use of antihypertensive, hypolipidemic hypoglycemic, antidepressants, and anticonvulsants agents); and laboratory (CD4 count prior to initiation of treatment).
Statistical analysisThe data were double entered and, in cases of discrepancies the medical records were reviewed. The statistical analysis was performed using STATA 12.0.
In a first step of the analysis we tested the association between the independent variables of each block of variables and ART modification. The magnitude of the association was expressed by the odds ratios and the statistical significance by the p-value and the 95% confidence interval.
The variables associated with the outcome with a p-value <0.20 in univariate analysis were included in multivariate analysis (multiple logistic regression) per block, followed by another stage of multivariate analysis, with the variables selected in each block. Variables associated with the outcome with p<0.05 remained in the final model.
To assess the time of using ART until modification due to adverse events and to compare different regimens we used the Kaplan-Meier survival curve, the long rank test and Cox proportional hazards analysis.
ResultsThe study sample consisted of 94 cases and 283 controls, who initiated first-line ART during the period from 2011 to 2014. The reasons for exclusion in this study are described in Fig. 1.
The study sample had 66.8% males, with a mean age of 38.4 years (95% CI: 37.3-39.5). There were more participants in the 18-35 age group, representing 44.3% of the sample, while the group aged over 50 years represented 14.1%.
On initiating ART, the median CD4 count was 278 cells/mm³ and 69% of participants presented with a CD4 count below 350 cells/mm³. Only 10.1% of participants presented with CD4 levels above 500 cells/mm³.
The majority of individuals initiated ART in the 2013-2014 period (52.3%). Between 2011 and 2012, 47.8% of participants initiated treatment with zidovudine, lamivudine and efavirenz and 36.7% with tenofovir, lamivudine and efavirenz, while 62.4% of participants started this regimen in the years 2013 and 2014.
Efavirenz was part of the first-line antiretroviral regimen for 88% of participants who initiated treatment between 2011 and 2014. The replacement rate of efavirenz due to adverse events amongst participants who started first-line ART in the study period was 19%, while for protease inhibitors (PI), initiated in only 12% of participants between the years 2011 and 2014, the replacement rate was 13%.
The median time elapsed between initiating treatment and the first modification due to adverse events was 70.5 days (95% CI=26-161 days). The minimum time to be register an adverse event was four days and the maximum time was 440 days.
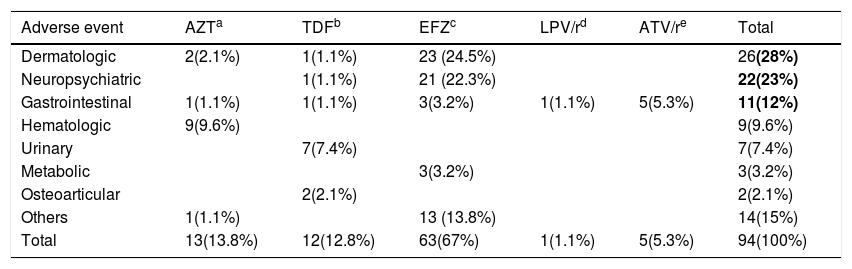
The main type of adverse events that led to ART modification was dermatologic in 28%; neuropsychiatric disorders in 23%; and gastrointestinal in 12% of the reports (Table 1).
Adverse events that led to modification of antiretroviral therapy in the Case Group.
| Adverse event | AZTa | TDFb | EFZc | LPV/rd | ATV/re | Total |
|---|---|---|---|---|---|---|
| Dermatologic | 2(2.1%) | 1(1.1%) | 23 (24.5%) | 26(28%) | ||
| Neuropsychiatric | 1(1.1%) | 21 (22.3%) | 22(23%) | |||
| Gastrointestinal | 1(1.1%) | 1(1.1%) | 3(3.2%) | 1(1.1%) | 5(5.3%) | 11(12%) |
| Hematologic | 9(9.6%) | 9(9.6%) | ||||
| Urinary | 7(7.4%) | 7(7.4%) | ||||
| Metabolic | 3(3.2%) | 3(3.2%) | ||||
| Osteoarticular | 2(2.1%) | 2(2.1%) | ||||
| Others | 1(1.1%) | 13 (13.8%) | 14(15%) | |||
| Total | 13(13.8%) | 12(12.8%) | 63(67%) | 1(1.1%) | 5(5.3%) | 94(100%) |
Bold values represent the main adverse events reported.
The main reported adverse events leading to modification of ART were allergic dermatitis, skin rash, urticaria, pruritus, and an episode of Stevens-Johnson syndrome in the group of dermatological events; dizziness, insomnia, drowsiness, headache, hallucination, and mood changes in the group of neuropsychiatric events; and vomiting, nausea, diarrhea, pancreatitis, and jaundice in the group of gastrointestinal events.
Dermatological symptoms were the earliest to appear in the first 15 days after initiating ART, representing 82.4% (14/17) of all adverse events in that period. Meanwhile, adverse events that occurred after six months of using ART involved the central nervous system (27.2%); urinary tract (22.7%), and gastrointestinal system (13.6%). A total of 71.4% of adverse events involving the urinary tract occurred six months after using ART.
The most frequently replaced drug at the time of the first adverse event was efavirenz, replaced in 67% of cases due to dermatological and neuropsychiatric events. Zidovudine was the second more frequently modified drug (14 %), in the first year of use, mostly due to hematologic events (anaemia and pancytopenia), and tenofovir, the third (13%) due to renal failure and osteopenia.
A total of 27 (7%) individuals who modified ART during the first year of treatment due to adverse events needed to modify therapy again. The main reasons were neuropsychiatric (nightmare, dizziness, drowsiness, and vivid dreams) in 38.5% of the cases and gastrointestinal (nausea, vomiting, diarrhea, and dyspepsia) in 34.6% of the cases.
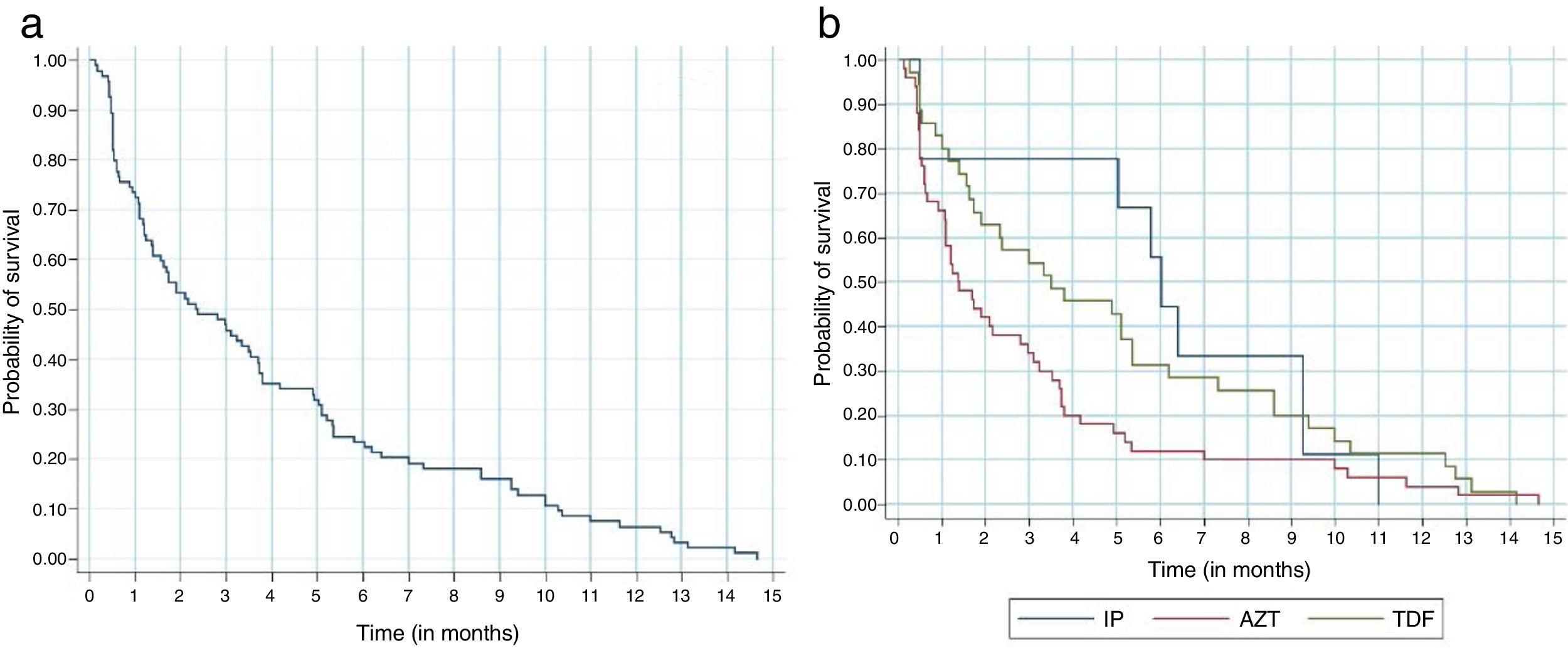
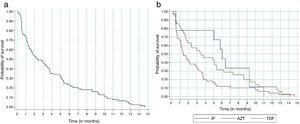
Regardless of the antiretroviral regimen used, survival analysis demonstrated that 30% of the participants who modified antiretroviral therapy for adverse events did so in the first month, and 80% modified until the sixth month of treatment (Fig. 2a). Participants taking tenofovir, lamivudine and efavirenz presented a 36% lower chance of ART modification due to adverse events when compared to those taking zidovudine, lamivudine and efavirenz (Fig. 2b).
The Kaplan-Meier survival curve on the modification of antiretroviral therapy in the first year of treatment due to adverse events. A: Survival analysis regardless of the antiretroviral regimen adopted. Probability of survival / Time (in months). B: Survival analysis according to the antiretroviral regimen used. Probability of survival / Time (in months). Log-rank – p=0.059 (three groups) / Log-rank– p=0.042 (AZT/EFZ x TDF/EFZ) / Cox Regression: HR 0.64 (0.41-0.99) p=0.045.
*IP: antiretroviral regimen with tenofovir and ritonavir-boosted protease inhibitors.
**AZT: antiretroviral regimen with zidovudine, lamivudine and efavirenz.
***TDF: antiretroviral regimen with tenofovir and efavirenz.
The Kaplan-Meier curve of the ritonavir-boosted protease inhibitors (PI/r) was atypical due to the lower number of participants initiating this antiretroviral drug in the first year of treatment. Approximately 55% of participants replaced the PI/r in the sixth month of treatment.
In addition, the PI/r were replaced much later in comparison to other regimens. This might have occurred for less severe or later onset adverse events, such as gastrointestinal complaints or metabolic abnormalities (Fig. 2b).
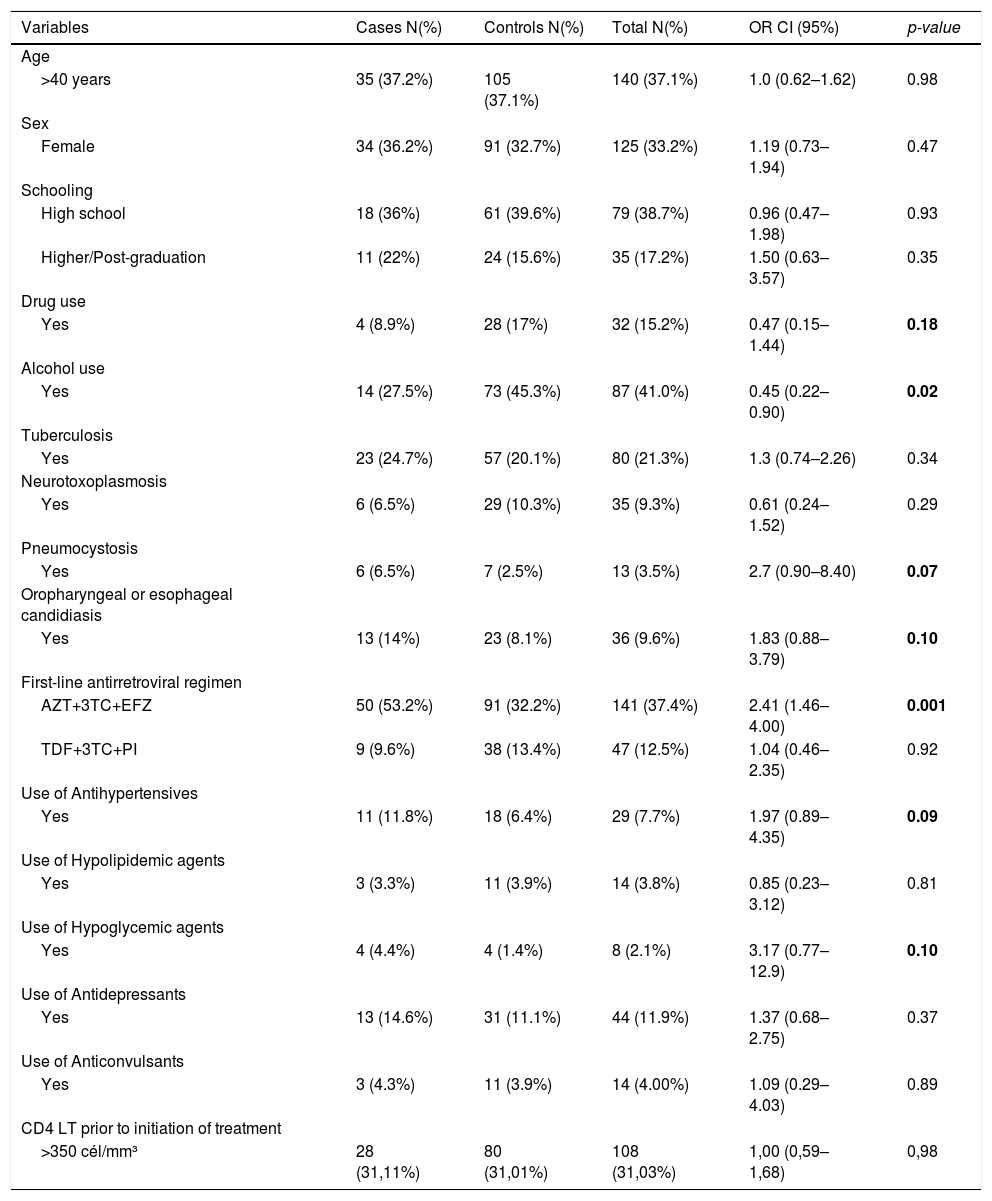
Factors associated with ART modification due to adverse events, identified in the univariate analysis with a p-value < 0.2 were: drug use; alcohol use; opportunistic infections in the first year of ART: tuberculosis, toxoplasmosis, pneumocystosis, oropharyngeal/esophageal candidiasis, cytomegalovirus, cryptococcosis/histoplasmosis; first-line regimen of zidovudine, lamivudine and efavirenz; use of antihypertensive and hypoglycemic agents (Table 2).
Suspension or modification of first-line antiretroviral therapy in HIV/AIDS participants, according to sociodemographic factors and life habits, clinical, therapeutic and laboratory factors in cases and controls attended at two public hospitals in the city of Recife-PE, between 2011 and 2014.
| Variables | Cases N(%) | Controls N(%) | Total N(%) | OR CI (95%) | p-value |
|---|---|---|---|---|---|
| Age | |||||
| >40 years | 35 (37.2%) | 105 (37.1%) | 140 (37.1%) | 1.0 (0.62–1.62) | 0.98 |
| Sex | |||||
| Female | 34 (36.2%) | 91 (32.7%) | 125 (33.2%) | 1.19 (0.73–1.94) | 0.47 |
| Schooling | |||||
| High school | 18 (36%) | 61 (39.6%) | 79 (38.7%) | 0.96 (0.47–1.98) | 0.93 |
| Higher/Post-graduation | 11 (22%) | 24 (15.6%) | 35 (17.2%) | 1.50 (0.63–3.57) | 0.35 |
| Drug use | |||||
| Yes | 4 (8.9%) | 28 (17%) | 32 (15.2%) | 0.47 (0.15–1.44) | 0.18 |
| Alcohol use | |||||
| Yes | 14 (27.5%) | 73 (45.3%) | 87 (41.0%) | 0.45 (0.22–0.90) | 0.02 |
| Tuberculosis | |||||
| Yes | 23 (24.7%) | 57 (20.1%) | 80 (21.3%) | 1.3 (0.74–2.26) | 0.34 |
| Neurotoxoplasmosis | |||||
| Yes | 6 (6.5%) | 29 (10.3%) | 35 (9.3%) | 0.61 (0.24–1.52) | 0.29 |
| Pneumocystosis | |||||
| Yes | 6 (6.5%) | 7 (2.5%) | 13 (3.5%) | 2.7 (0.90–8.40) | 0.07 |
| Oropharyngeal or esophageal candidiasis | |||||
| Yes | 13 (14%) | 23 (8.1%) | 36 (9.6%) | 1.83 (0.88–3.79) | 0.10 |
| First-line antirretroviral regimen | |||||
| AZT+3TC+EFZ | 50 (53.2%) | 91 (32.2%) | 141 (37.4%) | 2.41 (1.46–4.00) | 0.001 |
| TDF+3TC+PI | 9 (9.6%) | 38 (13.4%) | 47 (12.5%) | 1.04 (0.46–2.35) | 0.92 |
| Use of Antihypertensives | |||||
| Yes | 11 (11.8%) | 18 (6.4%) | 29 (7.7%) | 1.97 (0.89–4.35) | 0.09 |
| Use of Hypolipidemic agents | |||||
| Yes | 3 (3.3%) | 11 (3.9%) | 14 (3.8%) | 0.85 (0.23–3.12) | 0.81 |
| Use of Hypoglycemic agents | |||||
| Yes | 4 (4.4%) | 4 (1.4%) | 8 (2.1%) | 3.17 (0.77–12.9) | 0.10 |
| Use of Antidepressants | |||||
| Yes | 13 (14.6%) | 31 (11.1%) | 44 (11.9%) | 1.37 (0.68–2.75) | 0.37 |
| Use of Anticonvulsants | |||||
| Yes | 3 (4.3%) | 11 (3.9%) | 14 (4.00%) | 1.09 (0.29–4.03) | 0.89 |
| CD4 LT prior to initiation of treatment | |||||
| >350 cél/mm³ | 28 (31,11%) | 80 (31,01%) | 108 (31,03%) | 1,00 (0,59–1,68) | 0,98 |
Notes: 1. The totals for each of the factors studied varied, since participants with no information were excluded. 2. In first-line antiretroviral regimen, PI corresponds to LPV/r or ATV/r.
The variables that had p-value <0,2 in the univariate analysis are highlighted in bold.
In the multivariate analysis by block, the use of the antiretroviral regimen containing zidovudine (OR: 2.16 95% CI: 1.28-3.65 and p: 0.004) remained significant, even when adjusting for use of antihypertensive and hypolipidemic agents (Table 3).
Multivariate analysis per block of the therapeutic factors of significant variables in univariate analysis for suspending or modifying the first-line antiretroviral therapy in HIV/AIDS participants attended at two public hospitals in Recife, between 2011 and 2014.
| AZT+3TC+EFZ | 2.16 (1.28–3.65) | 0.004 |
|---|---|---|
| Antihypertensives | 1.46 (0.58–3.65) | 0.41 |
| Hypolipidemic agents | 2.62 (0.53–12.92) | 0.23 |
The variables that presented p-value <0,5 in the multivariate analysis is highlighted in bold.
The variables in the sociodemographic and life factors block were not associated with modification of ART due to adverse events. The use of alcohol and drugs did not remain in the final model of this block.
After multivariate analysis of the block of clinical variables, no association was found between opportunistic infections and modification of ART due to adverse events.
With regard to the therapeutic variables, there was an association between use of the regimen of zidovudine, lamivudine and efavirenz and modification of ART due to adverse events. The use of tenofovir, lamivudine and protease inhibitor regimen was not associated with modification of ART due to adverse events.
DiscussionFrom a universe of 2196 participants who initiated one of the four antiretroviral regimens recommended as first-line treatment by the Brazilian Ministry of Health between 2011 and 2014, 365 participants modified their treatment, 94 of whom due to adverse events. In this sample, 66.8% of the individuals were males, the mean age was 38.4 years. The median CD4 count was 278 cells/mm³. It was observed that 69% of the participants initiated treatment with a CD4 count less than 350 cells/mm³, hence with a diagnosis of AIDS. These characteristics were similar to what was described in the Brazilian Epidemiological Bulletin and in previous Latin America and Europe studies.9–11
Amongst the ART modifications during the first year of treatment, 25.7% occurred due to adverse events, and the median elapsed time between initiating ART and the first modification due to an adverse event was 70.5 days. The rate of ART modification due to adverse events was inferior to that described by Padua et al. (56.1%) amongst individuals who initiated treatment between 2001 and 2003,5 and Cardoso et al.7 (57.8%) for individuals treated between 1996 and 2006, both in Southeast of Brazil. In an Italian cohort, Biagio et al.11 identified a 19% modification rate of ART due to adverse events, more similar to the our study, amongst patients who initiated treatment between 2008 and 2014. One possible explanation for the different rates of ART modification due to adverse events among our study and the studies by Padua et al. and by Cardoso et al. could be the emergence of drugs with better toxicity profile compared to previously used drugs.3,7,11 The analysis of an observational cohort in the Netherlands reported a reduction of modifications in the first-line regimens comparing the periods between 2000-2005 and 2005-2010, probably due to an improvement in the profile of drugs used over the years. When comparing the two periods, there was a reduction from 161 events of treatment modifications due to adverse events to 90 events for 1000 person-years. However, adverse events remained the main reason for changing the first-line ART regimen.12
In the present study, the most frequent first-line regimen was tenofovir, lamivudine and efavirenz, and 62.4% of participants initiated treatment in the period between 2013-2014, reflecting the recommendations at that time. One of the favorable points for initiating treatment with tenofovir, lamivudine and efavirenz in Brazil was the introduction of a combination of these drugs in a single tablet in 2014.
The regimens used as first-line are different if we consider the year that treatment was initiated. When analyzing the ART request forms for 2008, Lima et al. reported that in the first-line regimen, 54% of participants were on zidovudine, lamivudine and efavirenz, while 44.1% took PIs. In a clinical cohort conducted in Rio de Janeiro from 1996 to 2006, the most prescribed antiretroviral regimen was zidovudine, lamivudine and efavirenz.10,13
Reports of adverse events depend on the type of antiretroviral prescribed. In the present study, the main adverse events leading to ART modification were dermatological, neuropsychiatric and gastrointestinal. Efavirenz was prescribed for 88% of individuals who initiated ART in the study period. For this group the modification rate during the first year was 19%, mostly related to the occurrence of dermatological and neuropsychiatric events. The preferential use of efavirenz was advocated by the World Health Organization (WHO) treatment guidelines in 2013, as well as by the Brazilian guidelines. Despite reports in the literature of neuropsychiatric events, amongst other adverse events that may occur, the advantages of efavirenz were related to the convenient dosage, which is administered as a single daily dose, the low risk of interaction with rifampicin, and the high efficacy in virological control.2,14,15
In general, neuropsychiatric symptoms may cease within the first few weeks after initiating treatment, although they can persist in the long term, interfering with adherence and virological control.15–17 In an African cohort of 2920 participants on efavirenz, 78% males and a mean age of 39 years, the incidence of neuropsychiatric events was 29.9 per 1000 participant-years, one third of whom presented with this event within 12 months of initiating ART.17 In the present study, neurological symptoms were the main complaint after six months of ART, and seemingly persisted or appeared even months after taking efavirenz.
Dermatological events after initiating ART may occur with any antiretroviral. However, reports are more frequent when NNRTIs are administered,18 and generally these adverse events appear earlier when compared to neuropsychiatric events caused by efavirenz. In our study, while 82.4% of the dermatological complaints occurred during the first 15 days of taking ART, these complaints accounted for only 4.5% of adverse events after six months of ART.
In the present study, 24.5% of the participants on efavirenz switched to another drug because of dermatological complaints, mainly cutaneous rash. One case of Stevens-Johnson syndrome was reported during the first 15 days after initiating ART. A cohort study of 15,396 patients in South Africa assessed the reasons for ART modification and also identified that the most commonly used regimen was tenofovir, lamivudine and efavirenz (73.9%). Efavirenz was prescribed for 87% (13,462) of patients in 2010-2013 and was discontinued in 29.38% of patients for hypersensitivity and in 29.34% of patients for neuropsychiatric adverse events.19
Another important class of antiretroviral are the PIs, which in this study were the second most modified, due to gastrointestinal events (13%). While these events are mainly related to the use of PIs, they may also occur with NRTIs, such as zidovudine.16,18 The different PI/r regimens used in other studies make it difficult to compare the data. Furthermore, there is divergence in the literature concerning the role of this drug class in the modification of ART due to adverse events. A study by Cardoso et al. in Rio de Janeiro reported a protective effect of NNRTIs and PI without ritonavir, with respect to adverse event ART modification, when compared to PI/r regimens. The most commonly used PIs in this study were nelfinavir and atazanavir, while the most commonly used NNRTI was efavirenz.7 Cicconi et al. evaluated modifications in ART between 1997 and 2007 and identified a relative risk of modifying the antiretroviral regimen due to adverse events of 1.66 for participants using PI/r when compared to those using NRTIs (p=0.0004).3
In relation to NRTIs, the first option in Brazil is tenofovir, justifying its use in 63% of first-line regimens of this study. Its replacement rate for adverse events was 5%, a lower rate compared to zidovudine, with renal adverse event being the main adverse event reported, often occurring after six months of ART. Between 2011 and 2014, zidovudine became the second-choice of NRTIs due to adverse events reported both in the short and long term.2 Myelosuppression is a potentially serious adverse event related to the use of this drug, which may occur during the first three months of using this drug. There is also a greater risk for those with baseline anemia, low CD4 count and being female.16,18–20 In our study, zidovudine was part of the initial antiretroviral regimen in 33% of participants and was modified in 9% of the cases, mainly due to hematological events such as anemia or myelosuppression
In our study, those on tenofovir, lamivudine and efavirenz were 36% less likely to modify their antiretroviral therapy due to adverse events during the first year of treatment than those who initiated treatment with zidovudine, lamivudine and efavirenz. These findings are consistent with other reports showing that tenofovir is well tolerated.11,16 Overall, tenofovir was better tolerated than zidovudine in our study. However, 5% of those on tenofovir had to change this drug due to renal or osteoarticular adverse events, a rate much higher than found in a meta-analysis involving more than 10,000 patients exposed to this drug.21 The higher replacement rate found may have short and long term implications as tenofovir is part of the first-line regimen.
We also evaluated the role of sociodemographic, life habits, and clinical and therapeutic factors in ART modifications due to adverse events. We did not find an association between sociodemographic factors and life habits and ART modification due to adverse events. Moreover, there is divergence in the literature concerning an association between sex or age and the occurrence of adverse events leading to ART modification, and several previous studies have made no mention of such an association.5,10,13,19 There is also few data in the literature regarding the role of alcohol and drugs in ART modification due to adverse events. Padua et al. evaluated alcohol use as a possible factor involved in the occurrence of adverse events, and found no association in univariate analysis.5 No other studies on modificatios of ART due to adverse events have evaluated alcohol use as a behavioral factor that could be associated with this outcome.4,7,11,13
After multivariate analysis, the only factor associated with ART modification due to adverse events was use of zidovudine containing regimen which is in agreement with previous studies that have demonstrated an increased risk of modifying ART due to adverse events in participants that have used NRTIs.5,11,19 The Italian cohort ICONA demonstrated that use of zidovudine was a predictor for ART modification due to adverse events and that individuals who used it were more likely to modify their therapy due to adverse events than those on tenofovir.11
With the current recommendations for early detection of HIV infection, initiating antiretroviral therapy irrespective of the CD4 count, and use of tenofovir in the first-line regimen, we would expect a reduction in the rate of ART modification due to adverse events. The use of antiretrovirals with better tolerance profiles and fewer adverse events may imply greater adherence to treatment and greater durability of first-line antiretroviral regimens, resulting in prolonged virologic suppression. Virological control is the major goal of treatment for people living with HIV/AIDS, avoiding the manifestations of acquired immunodeficiency and inhibiting immune activation and its consequences, guaranteeing quality of life for these individuals. On the other hand, virological control in HIV-infected people reduces transmission and therefore the number of new HIV infections, ultimately contributing to the control of the AIDS epidemic.3,12
In 2017, the Department of Sexually Transmitted Infections and AIDS in Brazil advocated a new antiretroviral regimen for the first-line treatment of HIV/AIDS participants, consisting of tenofovir, lamivudine and dolutegravir. The recommendation to use an integrase inhibitor in the first-line regimen is based on its efficacy in virological control, dosage convenience, and fewer reports of adverse events. Thus, efavirenz, the most commonly used and modified antiretroviral drug in our study due to adverse events, becomes a second therapeutic option for first-line treatment and is prescribed for participants with contraindications for dolutegravir.22
One potential limitation of this study was the collection of information from medical records, since several records lacked relevant information. To minimize this limitation, we retrieved complementary information from the national logistic system for antiretroviral control.
With the inclusion of new first-line antiretroviral drugs in Brazil and the expansion of treatment for all HIV/AIDS individuals, we would suggest to initiate an individualized regimen, taking into consideration clinical, laboratory and behavioral criteria. After ART initiation individuals should follow regular clinical and laboratory follow up consultations in order to identify potential adverse events. Furthermore, there is a need for a system to report adverse events related to antiretrovirals, including long term reactions. Preliminary data already indicate the occurrence of neuropsychiatric effects related to dolutegravir, a new first-line antiretroviral drug, with widespread use in distinct clinical trial scenarios.23
In conclusion, adverse events accounted for 25.7% of modifications of ART, and occurred with a median time of 70.5 days after initiating ART. The main adverse events were dermatological, neuropsychiatric and gastrointestinal. Dermatological events appeared earlier. Efavirenz was the most prescribed and most modified drug during the study period. Participants who used zidovudine/lamivudine/efavirenz had a 2-fold greater chance of switching ART due to adverse events when compared to those who used tenofovir/lamivudine/efavirenz.
Further prospective studies are needed to assess the role of other factors involved in modifying ART due to adverse events, such as the use of alcohol and illicit drugs, co-infection with hepatitis B or C, and the concomitant use of other medications for chronic comorbidities such as diabetes, hypertension, neuropsychiatric, and metabolic diseases common among people with HIV/AIDS.
Conflicts of interestThe authors declare no conflicts of interest.
Declarations of interestNone
This study was funded by Ministério da Saúde, Secretaria de Vigilância a Saúde (SVS) - Edital nº 20/2013, Estudos e Pesquisas Aplicadas em Vigilância em Saúde; and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Conselho Nacional de Desenvolvimento Científico e Tecnológico (309722/2017-9 to RAAX, 308590/2013-9 to DBMF).