This study aimed to review etiological and epidemiological data for hand, foot, and mouth disease (HFMD) cases that occurred between 2008 and 2010 in Guangzhou City, to help develop and implement precautionary measures applicable for future outbreaks.
MethodsThe characteristics of 4,753 HFMD episodes were retrospectively reviewed in 4,636 patients reported between 2008 and 2010 by the Guangdong Women and Children's Hospital, which is the national enterovirus monitoring agent and the designated hospital in China for treating severe HFMD.
ResultsOut of 4,753 incident episodes reviewed, 525 patients were hospitalized; 60% were males. Most patients (93.8%) were children under 5 years old, with a median age at onset of 2.4 years. HFMD incidence peaked in April/May and September/October. From the total, 1,067 (22.4%) infections were positive for human enterovirus 71 (HEV71), 1,094 (23.0%) were positive for coxsackievirus A16 (CA16), and 941 (19.8%) were positive for other common enteroviruses. In contrast, 1,666 (35.0%) cases were negative to HEV71, CA16, and other common enteroviruses. Cross-correlation coefficients demonstrated associations between the number of cases, seasonal temperatures, and humidity. Among hospitalized cases, HEV71 was positive in 261 (24.5%), and 42 (3.9%) critical cases were positive for HEV71.
ConclusionSeasonal fluctuations and HEV71 and CA16 were the two key factors influencing the Guangzhou HFMD epidemic. The infection predominantly affected children younger than 5 years old.
Hand, foot, and mouth disease (HFMD) is a human syndrome that arises from infection by intestinal viruses such as coxsackievirus A (CoxA) and human enterovirus 71 (HEV71), both members of the Picornaviridae family, genus Enterovirus.1 HFMD was first reported in New Zealand in 1957,2 and has since become quite frequent worldwide. HFMD is most prevalent in children; it is typically mild and self-limiting, and is moderately contagious, reaching epidemic proportions in close gatherings of young children, such as in day care centers and nursery schools or kindergartens.1,3,4 Transmission occurs from person to person through direct contact with the saliva, feces, vesicular fluid, or respiratory droplets of an infected person, or indirectly by contaminated articles. In recent years, several HFMD outbreaks occurred in China, Japan, New Zealand, Singapore, and Taiwan; children under 5 years old who are diagnosed with HFMD may subsequently develop encephalitis, encephalomyelitis, meningitis, pulmonary edema, circulatory failure, and other serious complications, even death.2–8
Numerous studies have shed light on the etiology and epidemiology of HFMD, and have helped medical professionals, government policy makers, and public health officials worldwide to advance their understanding of this condition. In Taiwan, Ho et al.4 assessed the epidemiologic aspects of an HFMD outbreak and found that HEV71 was associated with the most serious cases and nearly all of the deaths, most of which occurred as a result of pulmonary complications. Ang et al.3 reviewed the epidemiology of the 2000 Singapore epidemic and found that it was caused primarily by HEV71, although CoxA16 was also a cause of infection. Researchers concluded that HFMD was a significant public health problem and reported that the incidence per 100,000 individuals increased from 125.5 in 2001 to 435.9 in 2007; they urged prevention and control measures, especially in preschool centers. A review of the cases in Yokohama City, Japan, found that most samples were positive for enterovirus, and serotypes of poliovirus were also identified; the peak seasons were the warm summer months and early autumn, close to those reported in HFMD studies from other countries, and the infection was prevalent in children younger than six years old.7 Investigators recommended periodic surveys to monitor potentially severe diseases caused by enteroviruses. The Guangdong Women and Children's Hospital, as the national enterovirus monitoring agent, is particularly well prepared to investigate enteroviruses, because its laboratory regularly detects HEV71 and CA16 viruses as well as other common enteroviruses in large number of patients.
HFMD remains one of the most prevalent infectious diseases in China, including in the city of Fuyang of the Anhwei Province and in other regions, and affects up to one million individuals annually.8 Recently, Wang et al.9 reported that conditions for HFMD epidemics have persisted since March 2008 for almost 11 months of the year and that they show clear associations with the climate. The authors suggested that clustering was related first to geographic regions, and secondly to the amount of precipitation in the particular regions. The continuing epidemic of HFMD and the potential for serious complications has evoked the highest level of attention from the Chinese government. The disease was given communicable disease legal control status on May 2, 2008. Currently, however, little information is available to help determine whether significant differences exist in gender, age, or time of distribution among various viruses that have been known to cause HFMD. Additionally, although studies have analyzed the 2008 epidemic in China, patterns across these studies have not been determined. It is theorized that the analysis of related factors, such as seasonal distribution and peak incidence of cases, gender, age, and disease characteristics might reveal differences and patterns that could suggest early-stage interventions applicable to help impede disease progression.
The Guangdong Women and Children's Hospital is the designated hospital for treating severe HFMD, and hospital records show that the number of cases has been increasing. The annual incidence rate of HFMD per 100,000 people in China increased from 279.8 in 2008 to 743.8 in 2009,10 and this was reflected in the increased numbers of cases identified at this institution. Although other reports that explore the 2008 outbreak are available in the literature, it is theorized that supplementary analysis of the data could be valuable to further identify the HFMD epidemic characteristics, explore possible associations with seasonal variations in Guangzhou, and provide the government with a scientific basis for shaping policy, including public and professional health education to help prevent future outbreaks. Therefore, this study aimed to analyze the etiologic and epidemiologic features of the large number of HFMD cases reported to the Ministry of Health by this institution during a three-year period of the epidemic.
Patients and methodsEthical considerationsThis study was approved by the Ethics Committee and Institutional Review Board of Guangdong Women's and Children's Hospital of Guangdong Province, Guangzhou, China, on May 26, 2009.
PatientsA total of 4,753 HFMD cases in 4,636 patients diagnosed in Guangzhou City, China, were reported to the Chinese Ministry of Health between May 2008 and June 2010. This included 525 patients that required hospitalization for acute care, of whom 66 were critically ill and three died.
Survey methods4,753 reported cases of HFMD in 4,636 individuals were retrospectively reviewed, and all the data were analyzed. The epidemiological interpretation of distribution among people, location, and time (details shown below) was applied in this study for epidemiologic analysis and etiology of HFMD. All cases were evaluated according to specific diagnostic criteria described in a previous report,11 and disease classification was performed as defined by the Chinese Ministry of Health.10
Epidemiological interpretation of distributionTime distribution: analysis of the incidence of the HFMD was based on the growth characteristics of the causative viruses and the Guangzhou weather conditions over time (e.g., seasonal humidity, temperature, and precipitation), according to Guangzhou annual meteorological reports available online. Viruses survive and spread in humid, warm environments, and they are particularly sensitive to high temperatures, dryness, and ultraviolet radiation. Therefore, temperatures and humidity data in Guangzhou during the epidemic time period were statistically analyzed in relationship to the infection data (See statistical analysis for details).
Population distribution: age and gender distribution were evaluated to explore the cause of children's susceptibility. Most adults appear to be immune to the HFMD viruses and are asymptomatic carriers, able to spread the viruses to other persons, including their children.
Diagnostic criteriaAll cases were defined and evaluated based on previously published criteria,8 as follows:
- 1.
Clinically diagnosed cases had fever accompanied by rash of the hands, feet, lips, and buttocks; however, fever was not present in all cases and is not a mandatory criterion.
- 2.
Age is not a criterion. However, most cases are found in infants, preschool children, and young children.
- 3.
Clinical diagnosis depends primarily on patients’ positive tests for enteroviruses isolated and identified as CoxA16, HEV71, or other common enteroviruses known to cause HFMD. Real-time polymerase chain reaction (PCR) was used to identify the enteroviruses as previously reported.11 However, some patients with negative PCR results were diagnosed with HFMD based on symptoms alone.
- 4.
The levels of antibodies against CoxA16, EV716, or other common enteroviruses known to cause HFMD in acute and convalescent serum increased by over four times when compared to normal controls.
According to the guidelines for diagnosis and treatment of HFMD, issued by the Chinese Ministry of Health,10 patients were classified as 1) general cases, 2) severe cases, and 3) deaths, defined as follows:
- 1.
General cases: hand, foot, mouth, and hip rash, with or without fever. Symptoms may include painful ulcers in the mouth or throat, general malaise/fatigue, loss of appetite, diarrhea, and sometimes vomiting.
- 2.
Severe cases: (a) severe cases appear to have nervous system involvement, including apathy, sleepiness, vulnerability to shock, delirium, headaches, and vomiting; body tremors, myoclonus, nystagmus, ataxia, and eye movement disorders; inability to move or acute flaccid paralysis; convulsions; signs of meningeal irritation; and weakened or absent Achilles tendon reflex. (b) Critical cases include at least one of the following: frequent convulsions, coma, brain herniation, dyspnea, cyanosis, bloody phlegm, pulmonary rales on auscultation, or circulatory insufficiency (e.g., shock).
- 3.
Deaths: HFMD is the cause of death.
Data were expressed by number and percentage. The associations between categorical variables were analyzed by Fisher's exact test. Statistical significance was set at < 0.05. A three-stage time series approach was carried on to evaluate the impact of weather on HFMD rates. First, autoregressive integrated moving average (ARIMA) models were applied to HFMD cases, including the total number of infections and the detected numbers of the three viruses (CA16, HEV71, and other common enteroviruses) in a natural log scale. Natural log transformation was used to stabilize the time series. The best fitting model was automatically determined by the Statistical Package for Social Sciences (SPSS) 15.0 expert modeler, including ARIMA (0, 0, 1) for the total number of infections, ARIMA (0, 0, 1) for detecting the number of other common enteroviruses, and ARIMA (0, 1, 0) for detecting the number of CA16 and HEV71 viruses. Second, a cross-correlation function (CCF) was performed between the monthly average temperature and humidity versus the total number of cases and the detected numbers of the three viruses to determine the lag structures of temperature and humidity. Finally, the monthly average temperature and humidity were included in the multivariate ARIMA models according to the CCF, and the best fitting, most conservative model was considered as the final model. All statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc, Chicago, IL, USA).
ResultsA total of 4,753 HFMD episodes in 4,636 patients were reported during the study period (May 2008 to December 2010). The infective viruses, CA16, HEV71, or other common enteroviruses, were positively identified in 3,087 using real-time PCR. The common enteroviruses included coxasckievirus A types 4, 5, 9, and 10; coxasckievirus B types 2, 5, and 13; and echovirus types 4, 6, 9, and 11. None of these viruses was detected in 1,666 out the 4,753 cases.
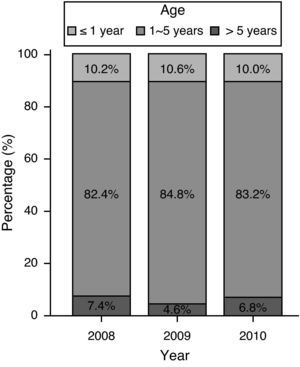
Age and gender distributionThe majority of onset ages ranged from one to five years; 10.2% of the participants were one year old or less; 6.2% were over 5 years old; and 83.6% were between 1 and five years. The median age at onset was 2.4 years. There were minor differences in age distribution in the period between 2008 and 2010 (p=0.02), including 526 (82.4%), 1,224 (84.8%), and 2,223 (83.2%) cases with age of onset between one and five years in 2008, 2009, and 2010, respectively. In addition, 47 (7.4%), 66 (4.6%), and 183 (6.8%) patients were over five years old in 2008, 2009, and 2010, respectively (Fig. 1). Among all the HFMD cases, 60.6% of the infections occurred in males. No significant differences in the gender distribution were observed throughout the three years (Table 1).
Summary of characteristics of infection cases.
| Year | p-value | |||
|---|---|---|---|---|
| 2008 (n=638) | 2009 (n=1443) | 2010 (n=2672) | ||
| Gender | ||||
| Female | 236 (37.0%) | 594 (41.2%) | 1,043 (39.0%) | 0.168 |
| Male | 402 (63.0%) | 849 (58.8%) | 1,629 (61.0%) | |
| CA16 positive | 147 (23.0%) | 541 (37.5%) | 406 (15.2%) | < 0.001* |
| HEV71 positive | 206 (32.3%) | 116 (8.0%) | 745 (27.9%) | < 0.001* |
| Positive for other common enteroviruses | 74 (11.6%) | 256 (17.7%) | 611 (22.9%) | < 0.001* |
| Critical cases | 1 (0.2%) | 14 (1.0%) | 51 (1.9%) | 0.001* |
| Hospitalization | 179 (28.1%) | 102 (7.1%) | 244 (9.1%) | < 0.001* |
| Deaths | 0 (0.0%) | 1 (0.1%) | 2 (0.1%) | 1.000 |
Of the total, 117 (2.5%) patients appeared to have had two separate infections during the study period; the median course interval between the two separate infections in a single patient was 9.5 months, ranging from 1.2 and 29.3 months. Of these 117 patients, 15 patients were negative for all three viruses in both infection episodes; 30 patients were positive to CA16, HEV71, or another enterovirus in only one of the two separate infection episodes; and 72 patients were positive for CA16, HEV71, or another common enterovirus in both infection episodes. Of these 72 patients, 27 appeared to be infected twice with the same virus(es), including CA16 alone in six patients, HEV71 alone in six patients, or another enterovirus alone in 14 patients, and CA16 and HEV71 together in one patient. Stool examination for the detection of intestinal virus-specific nucleic acid was performed to ensure that the second infection was not a repeated diagnosis (e.g., by antibodies) of the first infection. Furthermore, for patients who developed a second infection, the time elapsed from the first infection was observed for consistency with etiological results.
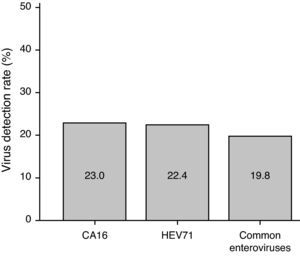
Virus infection ratesAmong 4,753 episodes, CA16 was detected in 1,094 infections (23.0%), including 1084 infections with CA16 only, nine infections with both CA16 and HEV71, and one infection with CA16 and other common enteroviruses. HEV71 was detected in 1,067 infections (22.4%), including 1,053 infections with HEV71 only, nine infections with both HEV71 and CA16, and five infections with HEV71 and other common enteroviruses. Common enteroviruses were detected in 941 infections (19.8%), including 935 infections with common enteroviruses only, five infections with both common enteroviruses and HEV71, and one infection with common enteroviruses and CA16 (Fig. 2). In contrast, 1,666 (35.0%) infections were negative to HEV71, CA16, and other common enteroviruses.
There were significant differences in the virus infection rate throughout the three years (all p<0.001) (Table 1). The detection rates for other common enteroviruses increased during the three-year period, from 11.6% in 2008 to 17.7% in 2009 and 22.9% in 2010. The CA16 detection rates were 23.0% in 2008, 37.5% in 2009, and 15.2% in 2010. The HEV71 detection rates were 32.3% in 2008, 8.0% in 2009, and 27.9% in 2010. However, the number of hospitalized patients did not increase corresponding to the increased HEV71 detection rate; in 2008, this hospital was the designated medical institution for critical care and rescue of HFMD patients and, therefore, the number of hospitalized patients increased. Also, HEV71 infection was not always an indicator of critical cases. In 2009, most cases were mild, requiring only treatment at the outpatient clinic or home isolation.
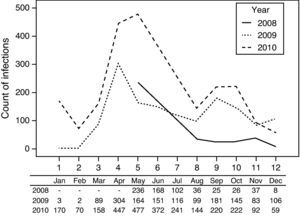
Monthly distributionThe onset peaks of HFMD in the three years occurred in May 2008, April 2009, and May 2010, respectively, and small onset peaks appeared in September and October in 2009 and 2010 (Fig. 3).
Critical cases and hospitalization for acute careA total of 66 critically ill HFMD cases were observed. The frequency of critical cases had a significantly increasing trend, including one (0.2%) case occurring in 2008, 14 cases (1.0%) occurring in 2009, and 51 cases (1.9%) occurring in 2010 (p<0.001) (Table 1).
A total of 525 infected patients were hospitalized for treatment, including 179 patients in 2008, 102 patients in 2009, and 244 patients in 2010. A significant difference was observed in the proportion of hospitalization between years: 28.1% in 2008, 7.1% in 2009, and 9.1% in 2010 (p<0.001).
MortalityThree HFMD-associated deaths were reported in 2009 and 2010 (Table 1). All of the fatal cases presented with prodromal fever, followed by mouth ulcers and rash on the hands, feet and rump; all three patients were HEV71-positive. Two males presented limb trembling during the earlier period, and the female had lip cyanosis, emesis, and lethargy. All three patients died of pneumorrhagia; two also had brainstem encephalitis, and the third one had pulmonary edema and severe pneumonia.
Associations between virus types and clinical characteristicsInfections by CA16 were associated with age (p=0.006); 8.2% of the patients with CA16 were younger than 1 year old, whereas 10.8% of those without CA16 were younger than one year old; 86.7% of the patients with CA16, but only 82.6% of the patients without CA16, had onset in the age range of 1 and 5. CA16 infection was associated with hospitalization for treatment and with critical cases. Patients with CA16 had a lower frequency of hospitalization and fewer of them were critically ill cases, when compared to patients without CA16 (9.1% vs. 11.6% hospitalized, p=0.021; 0.6% vs. 1.6% critically ill cases hospitalized, p=0.017) (Table 2).
Associations between virus type and clinical characteristics.
| CA16 | p-value | HEV71 | p-value | Common enteroviruses | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Detected | Not detected | Detected | Not detected | Detected | Not detected | ||||
| (n=1094) | (n=3659) | (n=1067) | (n=3696) | (n=941) | (n=3812) | ||||
| Age | |||||||||
| ≤ 1 year | 90 (8.2%) | 394 (10.8%) | 0.006* | 75 (7.0%) | 409 (11.1%) | < 0.001* | 117 (12.4%) | 367 (9.6%) | < 0.001* |
| 1-5 years | 949 (86.7%) | 3,024 (82.6%) | 912 (85.5%) | 3,061 (83.0%) | 787 (83.6%) | 3,186 (83.6%) | |||
| >5 years | 55 (5.0%) | 241 (6.6%) | 80 (7.5%) | 216 (5.9%) | 37 (3.9%) | 259 (6.8%) | |||
| Gender | |||||||||
| Female | 434 (39.7%) | 1,439 (39.3%) | 0.860 | 442 (41.4%) | 1,431 (38.8%) | 0.126 | 340 (36.1%) | 1,533 (40.2%) | 0.023* |
| Male | 660 (60.3%) | 2,220 (60.7%) | 625 (58.6%) | 2,255 (61.2%) | 601 (63.9%) | 2,279 (59.8%) | |||
| Hospitalization | 100 (9.1%) | 425 (11.6%) | 0.021* | 261 (24.5%) | 264 (7.2%) | < 0.001* | 88 (9.4%) | 437 (11.5%) | 0.072 |
| Critical cases | 7 (0.6%) | 59 (1.6%) | 0.017* | 42 (3.9%) | 24 (0.7%) | < 0.001* | 9 (1.0%) | 57 (1.5%) | 0.275 |
| Deaths | 0 (0.0%) | 3 (0.1%) | 1.000 | 1 (0.1%) | 2 (0.1%) | 0.534 | 0 (0.0%) | 3 (0.1%) | 1.000 |
Infections by HEV71 were associated with age (p<0.001); 7.0% of patients with HEV71 infections had onset before one year old, whereas 11.1% of those without infection by other common enteroviruses had onset before this age. HEV71 infection was associated with hospitalization for treatment and with critically ill cases. Patients with HEV71 had significantly higher proportions of hospitalization and critical cases than those without HEV71 infection (24.5 vs. 7.2% for hospitalization, p<0.001; 3.9% vs. 0.7% for critically ill cases, p<0.001) (Table 2).
Infections by other common enteroviruses were associated with age (p<0.001) (Table 2); 12.4% of the patients with these infections had onset before one year of age, whereas only 9.6% of the patients without infection by other common enteroviruses had onset before this age. Infections attributed to other common enteroviruses were associated with gender (p=0.023); 63.9% of the patients with other common enteroviruses were male, whereas only 59.8% of those without other common enteroviruses were male.
Among 4,753 infection cases, the causes of infections were confirmed in 3,087 cases, while the causes of the remaining 1,666 were unknown. The proportions of hospitalizations and critically ill cases increased significantly if the virus(es) was detected (14.3% vs. 5.0% for hospitalization, p<0.001; 1.8% vs. 0.7% for critically ill cases, p=0.001) (Table 3).
Association between the numbers of detected viruses and clinical characteristics.
| Characteristics | Virus detected (CA16, HEV71, and common enteroviruses) | p-value | |
|---|---|---|---|
| Not confirmed (n=1666) | Confirmed (n=3087) | ||
| Age category | |||
| ≤1 years | 202 (12.1%) | 282 (9.1%) | < 0.001* |
| 1-5 years | 1,340 (80.4%) | 2,633 (85.3%) | |
| >5 years | 124 (7.4%) | 172 (5.6%) | |
| Gender | |||
| Female | 661 (39.7%) | 1,212 (39.3%) | 0.78 |
| Male | 1,005 (60.3%) | 1,875 (60.7%) | |
| Hospitalization | 83 (5.0%) | 442 (14.3%) | < 0.001* |
| Critical cases | 11 (0.7%) | 55 (1.8%) | 0.001* |
| Deaths | 2 (0.1%) | 1 (0.0%) | 0.282 |
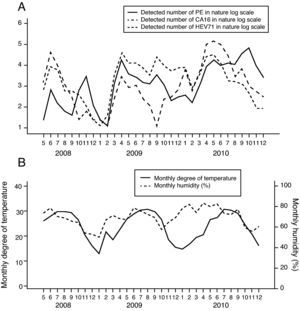
The trends of weather conditions and HFMD infections during the study period are presented in Fig. 4. Cross-correlation analyses show that the monthly temperature level was negatively correlated with the total number of HFMD cases (cross correlation -0.437) and the detected numbers of CA16 infections (cross correlation -0.455 and -0.446) (Table 4). In contrast, the monthly humidity was positively correlated with the total number of HFMD infections (cross correlation 0.536 and 0.651) and the detected numbers of other common enteroviruses (cross correlation 0.413) (Table 4).
Summary of cross correlation coefficients between the number of infections with temperature and humidity.
| Lag | log (Total) | log (CA16) | log (HEV71) | log (common enteroviruses) | ||||
|---|---|---|---|---|---|---|---|---|
| Cross correlation | SE | Cross correlation | SE | Cross correlation | SE | Cross correlation | SE | |
| Monthly temperature (oC) | ||||||||
| 0 | 0.26 | 0.18 | -0.035 | 0.18 | -0.332 | 0.18 | 0.252 | 0.18 |
| 1 | 0.255 | 0.183 | -0.228 | 0.183 | -0.351 | 0.183 | 0.224 | 0.183 |
| 2 | -0.109 | 0.186 | -0.455* | 0.186 | -0.344 | 0.186 | 0.002 | 0.186 |
| 3 | -0.271 | 0.189 | -0.446* | 0.189 | -0.312 | 0.189 | -0.222 | 0.189 |
| 4 | -0.437* | 0.192 | -0.289 | 0.192 | -0.111 | 0.192 | -0.361 | 0.192 |
| Monthly humidity (%) | ||||||||
| 0 | 0.536* | 0.18 | 0.342 | 0.18 | 0.163 | 0.18 | 0.361 | 0.18 |
| 1 | 0.651* | 0.183 | 0.014 | 0.183 | -0.051 | 0.183 | 0.413* | 0.183 |
| 2 | 0.341 | 0.186 | -0.32 | 0.186 | -0.18 | 0.186 | 0.318 | 0.186 |
| 3 | 0.252 | 0.189 | -0.323 | 0.189 | -0.239 | 0.189 | 0.259 | 0.189 |
| 4 | 0.035 | 0.192 | -0.314 | 0.192 | -0.178 | 0.192 | 0.144 | 0.192 |
The final models for HFMD episodes showed that one unit increase in monthly humidity was followed by a 0.08 unit increase in the total number of HFMD cases on the natural log scale after a time lag of one month (p<0.001); one unit increase in monthly humidity was followed by a 0.03 unit increase in the detected numbers of other common enteroviruses on the natural log scale after a one-month time lag (p=0.017); and one unit increase in monthly temperature levels was followed by a 0.051 unit decrease in the detected numbers of other common enteroviruses on the natural log scale after a one-month time lag (p=0.02) (Table 5). The model for HEV71 detection was not performed because no significant correlations between HEV71 detection and weather conditions (temperature and humidity) were observed in the cross-correlation function (Table 5).
Summary of the final models for HFMD infections.
| Lag | Estimate | SE | t | p-value | |
|---|---|---|---|---|---|
| logTotal: ARIMA(0,0,1) | |||||
| Constant | -0.961 | 0.885 | -1.086 | 0.287 | |
| MA(1) | -0.989 | 0.72 | -1.375 | 0.18 | |
| Monthly humidity (%) | 1 | 0.08 | 0.012 | 6.495 | < 0.001* |
| logCA16: ARIMA(0,1,0) | |||||
| Constant | 1.205 | 0.514 | 2.346 | 0.026* | |
| Monthly temperature degree | 2 | -0.051 | 0.021 | -2.462 | 0.020* |
| log common enteroviruses: ARIMA(0,0,1) | |||||
| Constant | 0.911 | 0.852 | 1.069 | 0.294 | |
| MA(1) | -0.991 | 1.845 | -0.537 | 0.595 | |
| Monthly humidity (%) | 1 | 0.03 | 0.012 | 2.532 | 0.017* |
The present review of the records of HFMD cases from Guangzhou City reported between 2008 and 2010 indicated that the main causative virus was CA16, but the more serious hospitalized or critically ill cases and deaths were all attributed to HEV71, and all deaths were associated with pulmonary complications. Differences in the virus detection rates over the three-year period were significant. The CoxA16 detection rates varied from year to year and were higher in 2009 (23.0% in 2008, 37.5% in 2009, and 15.2% in 2010). The HEV71 detection rates were 32.3% in 2008, 8.0% in 2009, and 27.9% in 2010. The detection rates for other common enteroviruses increased during the three-year period (11.6% in 2008, 17.7% in 2009, and 22.9% in 2010). The present findings were similar to other epidemiologic studies, particularly regarding the predominant population of children younger than five years old and the demonstrated association with seasonal weather (Fig. 4, Tables 4 and 5).
HFMD is a global infectious disease. Robinson et al.12 described recovering CA16 from stool samples and throat swabs of patients in Canada; serum antibody levels were four times higher in patient samples, ascertaining that CA16 was the first pathogen identified for this disease. Alsop et al.13 detected CA16 from herpes-infected patients in Birmingham in 1959, suggesting further that this virus was related to HFMD. HEV71 was first isolated in California in 1969 and it was subsequently associated with HFMD outbreaks in various countries.4,14 HEV71 was the primary infective organism in an HFMD epidemic in Taiwan in 1998, and since then HEV71 infection has become a serious public health issue associated with mortality among young children.15 However, CA2 was the predominant infective organism found in the 2008 outbreak in Taiwan.16
It should be noted that clinical diagnosis did not necessarily rely on laboratory findings; physicians, in some cases, made the HFMD diagnosis based on symptom presentation and not on positive identification of enteroviruses by PCR. As in other studies, the most serious cases with neurological complications were HEV71-infected children (18.6%), with only a small percentage (1.1%) of CA2-infected patients having such complications.16 Yang et al.17 confirmed that CA16 and HEV71 were the major pathogens linked to HFMD in 2002 in Shanghai with a CA16 to HEV71 ratio of 6.4:1. Zhao et al.18 confirmed that the ratio of CA16 to HEV71 in Beijing was 21:1 in 2007, and cases were primarily HEV71 in Fuyang in 2008.8 In Malaysia, echovirus was isolated from five patients with HFMD.19 In the present study, the ratio of CA16 to HEV71 in Guangzhou was 1:1.41 in 2008 and 1:1.66 in 2010. However, the ratio changed to 5.02:1 in 2009. The constituent ratio of positivity for HEV71 in 2009 (12.48%) was evidently lower than that reported in 2008 (44.51%) and 2010 (51.12%), consistent with the number of hospitalized cases in 2008 (29.24%) and in 2009 (only 4.65%). Regarding differences in virus detection in hospitalized patients by year, more cases were identified in the first year of the study (2008) because this hospital was serving as the critical care and rescue center for our province. In 2009, a training program for hospitals in nearby cities was started, which resulted in a decrease in the number of critical cases referred to this hospital. In 2010, a more severe outbreak occurred, which again increased the number of patients hospitalized due to enterovirus infections.
The present study found that HFMD infections occurring in Guangzhou showed a larger peak in April during the rainy season with high humidity and warmth, and a smaller peak in September and October in the period characterized by sunny weather with moderately warm temperatures. Both these peak periods are typically suitable for enterovirus growth and transmission. Sporadic cases also occurred in all other months except for January and February, which are exceptionally arid and have only 65 millimeters of average monthly rainfall and an average temperature of only 15.5o C, conditions that are not suitable for enterovirus growth. Urashima et al.20 tried to relate the seasonal aspects of HFMD incidence with global warming — or at least “urban warming” in Tokyo. They demonstrated correlations of 64% to outbreak of HFMD in the years 1999 to 2002. Their results suggest that warmer climates might lead to increased incidence of the viral disease. However, a study examining risk factors for enterovirus infection in children, especially HFMD, found that HEV71 infection occurred most often in geographic areas that were already associated with higher mortality rates, but not necessarily associated with weather conditions.21 Ma et al.22 examined the relationship between meteorological parameters and HFMD activity, and found that climate parameters were able to predict peak HFMD activity. The present study demonstrated an association between the number of HFMD cases identified and the seasonal weather conditions in the peak outbreak periods.
Regarding the prevalence of enteroviruses in young children, those under six months old still have maternal antibodies and, unlike older children, seldom have contact with each other. Therefore, this study and others1,3–5,8,19,22 found that the greatest percentage of patients falls in the age group between six months and five years old. Transmission has largely been attributed to intrafamilial and kindergarten/pre-school transmission during epidemics. Gender has also been noted in HFMD research; among all children with HFMD, males outnumbered females,3,15,23 but this finding has not yet been fully explained. Measures to help prevent outbreaks may include suspending classes that are experiencing many absences or closing schools altogether to stop disease transmission, as has been done in Singapore and Hong Kong.6 Public health surveillance and control are notable in Hong Kong, and HEV71 infection was declared a statutory notifiable disease in 2009, when other parts of China were also experiencing epidemic HFMD.
The present study has certain limitations. First, even though the study population was large, only HFMD patients treated in a single city and at a single institution were surveyed, which may limit the generalization of the results. Additionally, because research time and number of repeat cases were limited, no patients with more than two repeat infections were found in this study. However, since this hospital is the monitoring center for enterovirus, future surveillance will include continued monitoring of repeated infections.
In conclusion, this study suggested that HFMD outbreaks are definitely associated with seasonal weather patterns. The main causative virus is CA16, but most critical cases and deaths are attributed to HEV71. The present results helped clarify the etiology and epidemiology of the 2008 HFMD outbreak in Guangzhou City, which may in turn help in developing precautionary measures for future outbreaks and in curbing the increasing incidence of HFMD. Results from this study may facilitate the development and implementation of appropriate educational programs that could be delivered to the public in the months prior to seasonal outbreaks to help reduce the incidence of infection, especially among pre-school children. To ensure that HFMD outbreaks can be controlled, it is necessary to introduce public and professional health education initiatives, together with preventive measures, such as reduced contact between children, appropriate hygiene, and proper ventilation during the anticipated HFMD peak seasons. HFMD patients or their parents should be encouraged to accept treatment at home to avoid cross-contamination.
Conflict of interestAll authors declare to have no conflict of interest.
The authors would like to thank Mr. Changbin Zhang of the PCR Laboratory, Guangzhou Women and Children's Hospital and Health Institute, for his testing of the specimens. The authors also thank the staff in the Department of Pediatrics, Guangzhou Women and Children's Hospital and Health Institute for their help with discussion of the topic.
This study was supported by a grant from Guangzhou Women and Children's Hospital and Health Institute (2009 00165).