Increased serum CA 19-9 levels in patients with nonmalignant diseases have been investigated in previous reports. This study evaluates the clinical significance of serum CA 19-9 elevation in pulmonary nontuberculous mycobacterial disease and pulmonary tuberculosis. The median CA 19-9 level was higher in patients with pulmonary nontuberculous mycobacterial disease than in patients with pulmonary tuberculosis (pulmonary nontuberculous mycobacterial disease: 13.80, tuberculosis: 5.85, p<0.001). A multivariate logistic regression analysis performed in this study showed that Mycobacterium abscessus (OR 9.97, 95% CI: 1.58, 62.80; p=0.014) and active phase of pulmonary nontuberculous mycobacterial disease (OR 12.18, 95% CI: 1.07, 138.36, p=0.044) were found to be risk factors for serum CA 19-9 elevation in pulmonary nontuberculous mycobacterial disease. The serum CA 19-9 levels showed a tendency to decrease during successful treatment of pulmonary nontuberculous mycobacterial disease but not in pulmonary tuberculosis. These findings suggest that CA 19-9 may be a useful marker for monitoring therapeutic responses in pulmonary nontuberculous mycobacterial disease, although it is not pulmonary nontuberculous mycobacterial disease-specific marker.
Pulmonary nontuberculous mycobacterial (PNTM) disease appears to be increasing in many regions of the world, and the burden of PNTM is substantial.1–4 In South Korea and other regions in which the incidence of tuberculosis (TB) is intermediate, differential diagnosis between PNTM and TB has become an important issue as nontuberculous mycobacteria (NTM) isolation from respiratory specimens has increased.5,6
In PNTM disease, there is a lack of known biomarkers associated with disease activity and therapeutic response. Diagnosis of PNTM disease is defined by clinical criteria, radiographic presentation, and microbiologic results. Additionally, the goal of the treatment of PNTM disease includes symptomatic, radiographic, and microbiologic improvement. However, sputum specimen is frequently not achievable in some patients; the symptom profile of PNTM disease varies, and subjective assessment of the clinician has often come into play. Thus, many clinicians rely on radiography including chest X-ray and high-resolution computed tomography (CT) scanning to assess the severity of PNTM disease7 and for the monitoring of the treatment response. However, repeating CT scans increases cost and cumulative radiation dose. Thus, there is a need to identify biomarkers to estimate PNTM disease severity and to monitor treatment response.
Carbohydrate antigen 19-9 (CA 19-9) is a sialylated Lewis (Le) blood group antigen and a widely used tumor marker for epithelial type gastrointestinal cancers, especially pancreatic cancer.8,9 However, elevated levels of CA 19-9 can also be detected in patients with nonmalignant diseases including pancreatic, liver, and biliary diseases.10–12 In particular, there are several reports of increased serum CA 19-9 in several benign lung diseases including diffuse panbronchiolitis, emphysema, fibrosis, and bronchiectasis.12–14
A previous study reported that increased serum CA 19-9 levels may indicate clinical deterioration of PNTM disease15 and some reports suggest that elevated CA 19-9 levels decreased after successful treatment of PNTM disease, contrary to pulmonary tuberculosis.16–18
The aim of this study was to investigate the clinical significance of serum CA 19-9 elevation in PNTM diseases. We evaluated the factors associated with CA 19-9 elevation in PNTM disease and compared the change of CA 19-9 levels as a result of treatment of patients with either PNTM disease or TB patients.
Materials and methodsPatients and data collectionA total of 59 patients with PNTM disease and 36 patients with pulmonary TB who visited Severance Hospital, a university-affiliated tertiary referral hospital in South Korea, between March 2011 and December 2013 were enrolled. All patients provided written informed consent before enrollment and this prospective study was approved by the Ethics Review Committee of Severance Hospital. Any patients with active cancer within five years based on medical chart review and interview were excluded.
PNTM disease was diagnosed based on the American Thoracic Society (ATS) guidelines.19 Radiologic disease types of PNTM were categorized as nodular bronchiectatic (NB), fibrocavitary (FC), or mixed. Although the NB form was characterized with bronchiectasis and multiple centrilobular nodules, the FC form was defined as a fibrocavitary lesion on chest CT. The number of involved lobes was investigated as an indicator of radiological severity. NTM species were identified via a polymerase chain reaction (PCR)-restriction fragment length polymorphism method based on the rpoB gene.6,20 Among 59 patients with PNTM disease, 24 patients were treated with anti-NTM regimens according to the ATS guidelines.19 Active phase of PNTM disease was defined as present in patients with persisting positive microbiological cultures and clinical deterioration based on symptoms and radiographic findings, who was treated for PNTM disease or was advised to treat in six month.
Twenty-two patients with MAC (Mycobacterium avium-intracellulare) lung disease received a standardized antibiotic combination consisting of clarithromycin (1000mg/day), rifampicin (450mg for patients who were <50kg or 600mg for patients who were ≥50kg), and ethambutol (25mg/kg for two months, then 15mg/kg/day).19 Two patients with M. abscessus complex lung disease were treated with a standardized regimen consisting of intravenous cefoxitin (12g/day), amikacin (10–15mg/kg) and azithromycin (250mg/day).19 The treatment duration was usually 24 months including at least 12 months after sputum culture conversion.21
Response to anti-NTM treatment was defined as sputum culture conversion and radiographic improvement within 12 months of treatment. Culture conversion was defined as three consecutive negative sputum cultures from the date of the first negative culture after the start of anti-NTM treatment.21
The diagnosis of active pulmonary TB was based on positive respiratory specimen culture or the presence of caseating granulomas in lung tissue. Additionally, patients with negative mycobacterial culture but with high likelihood of active TB and overall good responses after TB treatment were included. TB patients who were lost to follow-up during anti-TB treatment period or had concomitant immunosuppressive diseases requiring therapy or clinical conditions (such as HIV infection, lymphoma) were excluded. Patients were classified as low or moderate/high risk according to the risk factors for relapse. The risk factors for relapse were as follows: (1) cavitary lesion on chest imaging at diagnosis and (2) positive sputum culture after two months of anti-TB treatment.22 The risk groups were classified according to the number of risk factors for relapse: two risk factors in the high risk group; one risk factor in the moderate risk group; and no risk factor in low risk group.
Measurement of serum CA 19-9Blood samples were separated via centrifugation and frozen immediately at −70°C. Serum CA 19-9 levels were measured with an Analytics E 170 (Elecsys module) immunoassay analyzer (Roche Diagnostics GmbH, Mannheim, Germany) using the electrochemiluminescence immunoassay technique. The normal range was defined as <34U/mL according to the manufacturer's instructions.
Serum C-reactive protein (CRP) was measured via Chemistry Autoanalyzer Hitachi 7600 (Hitachi Co., Japan) with Daiichi reagent (Daiichi-Hitachi) using the turbidoimmunometric assays (TIA) technique with the normal range defined as <0.30mg/dL according to the manufacturer's instructions. Serum CA 19-9 was measured before treatment in all patients and also measured again after at least 12 months of treatment in 24 patients with PNTM disease and after the completion of treatment in all TB patients.
Statistical analysisCategorical variables were analyzed using the χ2 test and continuous variables were analyzed using the Mann–Whitney test. The non-parametric Wilcoxon signed-rank test was used for comparisons before and after treatment. Multivariate logistic regression analysis was performed to evaluate the factors related to CA 19-9 elevation. Variables with p<0.1 in the univariate analysis were entered into a multiple logistic regression model. All data were analyzed using GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA) and SPSS v. 20.0 (SPSS Inc., Chicago, IL, USA). A p-value <0.05 was considered to indicate significance.
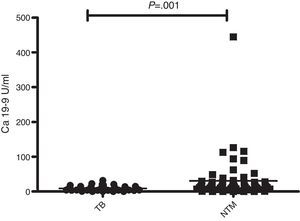
ResultsComparisons between patients with PNTM disease and patients with pulmonary TBThe baseline clinical characteristics of the patients are presented in Table 1. The proportion of female patients was higher among patients with PNTM disease than in patients with pulmonary TB. The median age of the patients with PNTM disease was higher than in patients with pulmonary TB (PNTM: 63, TB: 26.5, p<0.001). Radiologic severity defined by extension into more than three lobes was more frequent in patients with PNTM disease, but cavitary lesions were similar between the two groups. The proportion of subjects with AFB positivity and previous TB history was higher in patients with PNTM disease than in patients with pulmonary TB.
Clinical characteristics of patients with pulmonary nontuberculous mycobacterial (PNTM) disease and active tuberculosis (TB).
| PNTM disease(n=59) | TB(n=36) | p-Value | |
|---|---|---|---|
| Sex, female | 45 (76.3%) | 18 (50%) | 0.009 |
| Agea | 63 (43, 84) | 26.5 (22, 69) | <0.001 |
| BMIb | 20 (18.0, 22.0) | 19.72 (18.8, 21.9) | 0.664 |
| Smoking presence | 0.066 | ||
| Never | 49 (83.1%) | 24 (66.7%) | |
| Ever | 10 (16.9%) | 12 (33.3%) | |
| Past TB | 21 (35.6%) | 1 (2.8%) | <0.001 |
| AFB smear positive | 12 (20.3%) | 3 (8.3%) | 0.153 |
| Extension more than three lobes | 28 (47.5%) | 3 (8.3%) | <0.001 |
| Cavity | 21 (35.6%) | 11 (30.6%) | 0.614 |
| 2-Month culture (+) after TB treatment | – | 1 (2.8) | |
| Extrapulmonary TB | – | 3 (8.4) | |
| Drug resistant TB | – | 3 (8.4) | |
PNTM disease: pulmonary nontuberculous mycobacterial disease, TB: tuberculosis, BMI: body mass index, AFB: acid fast bacilli.
The median CA 19-9 level was higher in patients with PNTM disease than in patients with pulmonary TB (PNTM: 13.80, TB: 5.85, p<0.001, Fig. 1).
Comparisons between patients with normal and elevated CA 19-9 levels in PNTM diseaseThe CA 19-9 levels of 59 patients with PNTM disease were measured before NTM treatment; these patients were divided into two groups: ‘Normal CA 19-9 levels’ and ‘Elevated CA 19-9 levels’ (Table 2). The subjects with ‘Elevated CA 19-9 levels’ were all females. Body mass index (BMI), radiologic disease type, and serum CRP levels did not differ between the two groups.
Characteristics of patients with pulmonary nontuberculous mycobacterial (PNTM) disease according to the CA 19-9 level.
| Normal CA 19-9(n=48) | Elevated CA 19-9(n=11) | p-Value | |
|---|---|---|---|
| Sex, female | 34 (70.8%) | 11 (100%) | 0.036 |
| Agea | 64 (43, 84) | 60 (46, 76) | 0.067 |
| BMIb | 20.1 (18.6, 22.3) | 19.9 (17.9, 23.5) | 0.977 |
| Smoking | |||
| Never | 38 (79.2%) | 11 (100%) | 0.252 |
| Ex-smoker | 8 (16.7%) | 0 (0%) | |
| Current | 2 (4.2%) | 0 (0%) | |
| Past TB history | 13 (27.1%) | 7 (63.6%) | 0.027 |
| AFB smear positivity | 10 (20.8%) | 2 (18.2%) | 0.606 |
| Brochiectasis | 45 (93.8%) | 9 (81.8%) | 0.23 |
| Radiologic disease type | |||
| Nodularbronchiectatic (NB) | 30 (62.5%) | 8 (72.7%) | 0.76 |
| Fibrocavity (FC) | 1 (2.1%) | 0 (0.0%) | |
| NB+FC form | 17 (35.4%) | 3 (27.3%) | |
| Radiologic severity | |||
| >3 lobes | 19 (39.6%) | 9 (81.8%) | 0.013 |
| ≤3 lobes | 29 (60.4%) | 2 (18.2%) | |
| Main species | 0.007 | ||
| MAC | 37 (77.1%) | 3 (27.3%) | |
| M. abscessus complex | 4 (8.3%) | 2 (18.2%) | |
| MAC+M. abscessus complex | 6 (12.5%) | 6 (54.5%) | |
| M. kansasii | 1 (2.1%) | 0 (0%) | |
| PNTM disease treatment | 16 (33.3%) | 8 (72.7%) | 0.037 |
| Active phase of PNTM disease | 18 (37.5%) | 10 (90.9%) | 0.002 |
| Sputum culture conversionc | 9 (37.5%) | 3 (37.5%) | >0.95 |
| CRP (mg/dL)b | 0.11 (0.06, 0.33) | 0.1 (0.06, 0.19) | 0.633 |
MAC: Mycobacterium avium-intracellulare, BMI: body mass index, TB: tuberculosis, AFB: acid fast bacilli.
Through univariate analysis, extensive pulmonary lesions involving more than three lobes (p=0.021), M. abscessus (p=0.002), previous TB history (p=0.028), and active phase of PNTM disease (p=0.010) were associated with elevated CA 19-9 levels (Table 3). In a subsequent multivariate logistic analysis, Mycobacterium abscessus (OR 9.97, 95% CI: 1.58, 62.80; p=0.014) and active phase of PNTM disease (OR 12.18, 95% CI: 1.07, 138.36, p=0.044) were associated with an elevated CA 19-9 levels (Table 3).
Factors associated with elevated CA 19-9 levels in patients with pulmonary nontuberculous mycobacterial (PNTM) disease.
| CA 19-9≥34U/mL | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.94 (0.78, 1.02) | 0.118 | – | – |
| BMI | 0.99 (0.79, 1.24) | 0.946 | – | – |
| Past TB history | 4.71 (1.18, 18.79) | 0.028 | 2.64 (0.44, 15.72) | 0.286 |
| Cavity | 0.63 (0.15, 2.66) | 0.525 | – | – |
| AFB positivity | 0.84 (0.16, 4.55) | 0.844 | – | – |
| Extension more than half | 6.87 (1.34, 35.3) | 0.021 | 2.75 (0.37, 20.51) | 0.323 |
| M. abscessus complex | 10.13 (2.26, 45.35) | 0.002 | 9.97 (1.58, 62.79) | 0.014 |
| Active phase of PNTM disease | 16.67 (1.97, 141.24) | 0.010 | 12.18 (1.07, 138.36) | 0.044 |
BMI: body mass index, TB: tuberculosis, AFB: acid fast bacilli, M. abscessus complex: Mycobacterium. M. abscessus complex includes M. abscessus and M. massiliense.
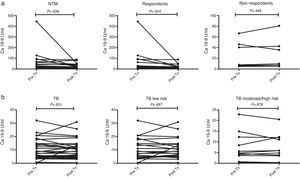
Serial CA 19-9 measurements were obtained in 24 patients with PNTM disease and 36 patients with pulmonary TB (Fig. 2).
Trend of CA 19-9 levels before and after treatment in patients with pulmonary nontuberculous mycobacterial (PNTM) disease (a) and patients with active TB (b). NTM, nontuberculous mycobacterial; TB, tuberculosis. Respondents: patients who had sputum culture conversion and radiologic improvement after anti-NTM treatment within 12 months, non-respondents: patients who had persistent positive sputum culture and aggravated radiologic manifestation despite anti-NTM treatment.
Although all patients with pulmonary TB showed sputum conversion to negative (culture positive cases) and clinical improvement (clinically diagnosed TB cases) after anti-TB treatment, patients with PNTM diseases were divided into respondents (n=17) and non-respondents (n=7) based on the response to anti-NTM treatment. Although the respondents showed sputum culture conversion and radiologic improvement after anti-NTM treatment within 12 months, the non-respondents had persistent positive sputum culture and aggravated radiologic manifestation despite anti-NTM treatment. The TB patients were classified into the low-risk (n=25) and moderate-/high-risk (n=11) groups based on risk factors for relapse.
The trend of serum CA 19-9 levels of the patients with PNTM disease after treatment was different according to the response to treatment (Fig. 2a). In the respondent group, the serum CA 19-9 level decreased significantly from a median of 18.50U/mL (IQR, 3.35–75.75) before treatment to 14.20U/mL (IQR, 3.90–22.10) after treatment (p=0.004). In comparison, the change in serum CA 19-9 levels before and after treatment in the non-respondents group was not significant (Pre-Tx: 7.80U/mL; IQR, 6.10–46.10; Post-Tx: 9.70U/mL, IQR, 5.10–42.60; p=0.446).
Interestingly, the trend in serum CA-19-9 levels of the patients with pulmonary TB was different from those of the PNTM patients (Fig. 2b). The levels of serum CA 19-9 were not significantly different between the low-risk and moderate-/high-risk groups either before or after anti-TB treatment. Additionally, the serum CA 19-9 levels before and after anti-TB therapy were not significantly affected by the risk factors of relapse.
DiscussionCA 19-9 is a glycosphingolipid of the Lewis group that is well known as a tumor-associated antigen.8 It is a useful marker of epithelial gastrointestinal cancers and pancreatic cancer. Recently, increased concentrations of serum CA 19-9 have been reported in various non-malignant lung diseases including bronchiectasis and emphysema.12 In particular, there are some reports of increased CA 19-9 levels in NTM lung disease.16,17 Our study is the first to evaluate the clinical meaning of elevated CA 19-9 in patients with PNTM disease.
CA 19-9 is synthesized and secreted by normal central airway and respiratory glands.23 Additionally, serum CA 19-9 is elevated by the extravasation of mucus glycoprotein that is hypersecreted from hypertrophic gland and epithelial cells in bronchioles.13,16,24
In our study, we showed the significant difference in CA 19-9 levels between patients with PNTM disease and patients with pulmonary TB. Whereas 18.6% of the patients with PNTM disease showed elevated serum 19-9, none of the patients with pulmonary TB did.
The reason why CA 19-9 is higher in patients with PNTM disease than in patients with pulmonary TB may be that chronic inflammation accompanying the bronchiectasis is more common in patients with PNTM disease than in those with pulmonary TB. Similarly, Kim et al. suggest that the mechanism responsible for increased CA 19-9 in benign pulmonary disease could be an inflammatory process in bronchiectasis, bronchiolitis, interstitial fibrosis, and emphysema.12 There are limitations to the use of CA 19-9 as a PNTM disease-specific marker, considering that CA 19-9 concentrations can also be increased in patients with other benign lung diseases. Therefore, the CA 19-9 levels might be interpreted by the degree of the inflammatory activity, not by the etiologic agent.
In practice, it is important to interpret the clinical meaning of the elevated CA 19-9 in patients with PNTM disease without cancer. In our study, the level of CA 19-9 was higher in patients with active phase of PNTM disease and those with M. abscessus infection.
The initial CA 19-9 level could be the marker reflecting inflammatory activity and guide the initiation of therapy in PNTM disease, although it is not a PNTM disease-specific marker.
Furthermore, the CA 19-9 might be a useful tool for monitoring treatment response in PNTM disease. Our data show that a decrease in CA 19-9 levels after anti-NTM treatment may reflect culture conversion and radiological improvement. On the other hand, the levels of CA 19-9 did not decrease in patients with pulmonary TB, regardless of risk factors for relapse, although all patients were cured.
This result is consistent with those of previous studies. Yamazaki et al. showed that CA 19-9 was a marker for the deterioration of a MAC infection in association with BMI and CRP.15 Tasci et al. showed that CA 19-9 does not change significantly after treatment in the patients with pulmonary TB in contrast with CA 125 and CA 15-3.18 Watanabe et al. found that CA 19-9 and immunoglobulin A (IgA) are significantly higher in patients with MAC infection than in patients with pulmonary TB and explained that these results might suggest that MAC infection leads to impaired airway defense.25
Although the level of serum CA-125 reflects the activity and severity in both pulmonary TB and PNTM disease,26,27 our data suggest that CA 19-9 is useful as a marker in the setting of PNTM disease but not in pulmonary TB. It is not clear whether this result may originate from the difference of inflammatory pathogenesis between TB and PNTM disease. The clinical significance of each tumor marker vis-à-vis a specific benign disease has yet to be defined, although the relevance between several tumor markers and inflammation are known.28 Because the trend of CA 19-9 according to clinical course was analyzed in a small number of patients, further studies in a larger cohort study involving PNTM diseases are needed.
The discrepancy of CA 19-9 levels among patients infected with different species in PNTM disease has not been investigated. M. abscessus infection constituted a significant factor associated with the elevation of serum CA 19-9 in PNTM disease in our study. However, we have not assessed the association between M. abscessus infection and CA19-9 levels. There is substantial overlap in the radiographic patterns of M. abscessus infection and the nodular bronchiectatic form of M. avium-intracellulare (MAC) infection, although thin-walled cavity and volume loss are found more frequently in the setting of MAC infection.19,29 Therefore, there may be other mechanisms beyond the bronchiectasis-related inflammatory process that mediate inflammation.
This study has several limitations. First, no healthy control group was evaluated. The study was designed to evaluate factors related to elevated CA 19-9 levels and changes in CA 19-9 level after treatment of PNTM disease and pulmonary TB. Therefore, we measured CA 19-9 levels in patients with PNTM disease and patients with pulmonary TB, without a control group. Secondly, only 24 of 59 patients with PNTM disease underwent treatment and serial blood sampling. This was because the diagnosis is not connected to the treatment in PNTM disease in contrast to pulmonary TB. Finally, the number of participants with PNTM and TB was small.
In conclusion, serum CA 19-9 levels were higher in patients with PNTM disease than in TB patients. CA 19-9 elevation was associated with active phase and M. abscessus infection in PNTM disease. In addition, the CA 19-9 levels showed a tendency to decrease during successful treatment of PNTM disease unlike pulmonary TB. Therefore, CA 19-9 may be useful in the evaluation of activity and therapeutic responses in patients with PNTM disease, although it is not a PNTM disease-specific marker.
Ethical standardThe study was carried out under the permission of the Ethics Review Committee of Severance Hospital.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by a National Research Foundation of Korea grant funded by the Korean Government (2011-0013018).