Retrospective clinical data suggest that antibiotic combinations, including tigecycline (TIG) and polymyxin (POL), result in better outcomes than monotherapy against Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae (KPC-KP).1 An in vitro study with time-kill assay has shown that TIG, POL and meropenem (MPN) as single agents do not exhibit efficient bactericidal activity against most of the KPC-producing strains, and TIG alone might be a therapeutic option for infections caused by KPC-producers when bacteriostatic activity is adequate, or combined with POL when bactericidal activity is required. The TIG and MPN association was neither synergistic nor bactericidal against KPC-KP strains, suggesting an antagonist effect, as demonstrated by Pournasara et al. in a previous publication in the IJAA.2 Additional in vivo tests are warranted to better assess killing kinetics of TIG in combination with other antibiotics against KPC-producers.
After approval by the local Ethics Committee for Animal Experimentation, a non-lethal experimental murine model of KPC-KP sepsis was conducted, aiming to observe the effect of different monotherapies and antimicrobial combinations on blood cultures and quantitative peritoneal cultures. Thirty-six rats were inoculated with a low dose inoculum (9×108CFU/mL) of a KPC-KP. MPN minimal inhibitory concentration (MIC) was above 16μg/mL, but the strain was susceptible to TIG (MIC=1μg/mL), POL B (MIC<0.5μg/mL) and gentamicin (MIC=4μg/mL). Antibiotic dosages and combinations are described in Table 1. Dosages were the same in combined and monotherapy groups. A control group without antibiotic was included.
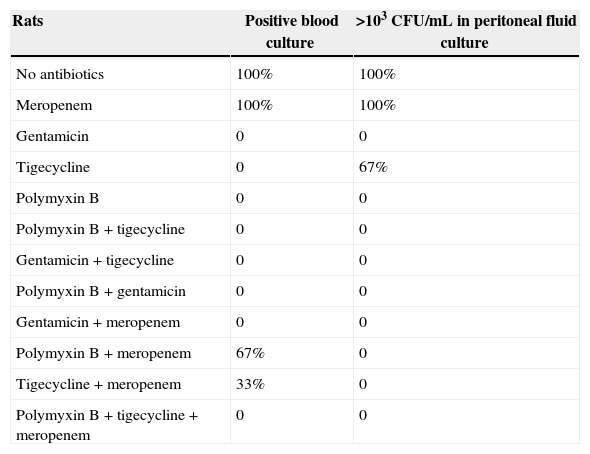
Experimental study with non-lethal sepsis in rats by KPC-producing Klebsiella pneumoniae. Monotherapy was compared with antibiotic combinations. Meropenem combined with other antibiotics besides having no benefit, positive cultures suggest an antagonistic effect.
| Rats | Positive blood culture | >103CFU/mL in peritoneal fluid culture |
|---|---|---|
| No antibiotics | 100% | 100% |
| Meropenem | 100% | 100% |
| Gentamicin | 0 | 0 |
| Tigecycline | 0 | 67% |
| Polymyxin B | 0 | 0 |
| Polymyxin B+tigecycline | 0 | 0 |
| Gentamicin+tigecycline | 0 | 0 |
| Polymyxin B+gentamicin | 0 | 0 |
| Gentamicin+meropenem | 0 | 0 |
| Polymyxin B+meropenem | 67% | 0 |
| Tigecycline+meropenem | 33% | 0 |
| Polymyxin B+tigecycline+meropenem | 0 | 0 |
CFU/mL, colony-forming units per milliliter.
No differences were observed in untreated controls and animals treated with MPN, since both groups had positive blood cultures and peritoneal fluid cultures with 103–104colony-forming units/mL (CFU/mL). All animals treated with TIG, GEN and POL as monotherapy had negative blood cultures, as did the animals treated with POL plus GEN, POL plus TIG, TIG plus GEN and triple therapy. Those groups also presented negative peritoneal fluid cultures, except for two animals treated with TIG monotherapy, who had 103CFU/mL in peritoneal fluid cultures. When POL and TIG were combined with MPN, three out of six blood cultures turned out positive after 12h of incubation, a worse microbiological result than monotherapies with TIG, POL and GEN and other combinations.
All monotherapies with TIG, POL and GEN (total n=9) showed higher efficacy in sterilizing peritoneal and blood cultures than the group of untreated controls and animals treated with MPN (n=6) (p=0.011). Triple therapy (MPN+TIG+POL) and double therapies with no MPN (TIG+POL, TIG+GEN, POL+GEN) (total n=12) were significantly more effective than controls (untreated controls and animals treated with MPN alone) in sterilizing cultures (p=0.011). On the other hand, when the TIG+MPN and POL+MPN combinations (n=6) were compared to untreated controls and animals receiving MPN (n=6), there were no significant differences in culture positivity (p=0.275).
This finding must be confirmed with larger samples, but these data suggest that combinations of MPN with TIG or POL might have an antagonist effect in vivo.
As previously reported by Pournarasa, 2011, MPN plus TIG was non-synergistic in this in vivo experiment. Additionally, POL plus MPN or TIG plus MPN associations may be less effective than monotherapies or other combinations to treat KPC-KP sepsis.
Antibiotic associations should be cautiously used, even for carbapenemase-producing Enterobacteriaceae strains. In vitro studies showing this paradox or antagonist effects used empirical combination therapies in humans with infections by KPC-KP. An experimental study with concentrated inoculum aiming to evaluate the effect of antimicrobial combination in lethal sepsis is ongoing and may elucidate this subject. Clinical trials could answer those questions, but, until then, the combined therapy for synergistic effect of carbapenems and other classes of antimicrobials is not recommended, at least for strains with higher MPN MIC.
Conflicts of interestThe authors declare no conflicts of interest.
Authors acknowledge the staff of Animal Laboratory of Universidade Estadual de Ponta Grossa.