Cytomegalovirus end-organ-disease (CMV EOD) is still a major cause of debilitating illness in people living with HIV, especially in developing countries.
ObjectiveTo evaluate the efficacy and safety of preemptive therapy against CMV EOD in HIV-positive adults with CMV viremia.
MethodsSystematic review of clinical trials by searching electronic databases and clinical trial registries, screening and selection of references, data extraction and assessment of risk of bias. The results were presented in a narrative synthesis. Aggregated analyzes for dichotomous outcomes were reported as odds ratios with 95 % Confidence Intervals.
ResultsFour RTC were included. A reduction in the risk of CMV EOD with preemptive therapy was found OR=0.49 (95 % CI 0.31‒0.76). We did not identify significant differences for all-cause mortality, adverse events, and withdrawal of the therapy secondary to adverse events.
ConclusionsPreemptive therapy could be a potential option for preventing CMV EOD in people living with HIV.
CMV is a fairly common virus worldwide, whose seroprevalence is close to 60 % in developed countries and more than 90 % in developing countries.1,2 Infection is latently established in body fluids and persists throughout the rest of life. In immunocompromised subjects, the virus can reactivate its replication and generate constant or intermittent viremia, increasing the risk of developing end organ disease.3,4
Before the introduction of highly active antiretroviral therapy (HAART), approximately 40 % of HIV-positive adults were diagnosed with CMV EOD.3,5 Along with the expansion of HAART, the incidence of this condition has decreased by 75 %. However, it remains a major cause of debilitating disease, especially in those diagnosed in very advances states with CD4 cell count <200 cells/mm3 or WHO stage 3 or 4, favoring a delayed initiation of HAART.4 Studies conducted in Ghana 6 and Tanzania 7 in HAART naive patients, have shown a prevalence of CMV infection of 16.7 % and 22.6 %, respectively. In Spain, an increment in the incidence density from 0.6 cases per 1000 person-years from 2004 to 2010 to 4.5 cases per 1000 person-years from 2010 to 2015 has been reported.8
Preemptive therapy consists of the administration of antiviral prophylaxis when CMV infection is diagnosed, in the absence of related EOD. This therapy is recommended in international consensus for patients receiving hematopoietic cell transplants and solid organ transplants to prevent the occurrence of CMV EOD, with clinical evidence supported by several randomized studies.5,9 However, there is not a recommendation in favor to the use of preemptive therapy in HIV positive people, with concerns about the utility and safety profile of the antivirals in this scenario. Therefore, the objective of this systematic review is to evaluate the efficacy and safety of CMV EOD preemptive in people living with HIV.
Materials and methodsProtocolThe protocol was registered in the International Prospective Registry of Systematic Reviews (PROSPERO): CRD42022326673.
Search strategyWe developed a search strategy to identify as many Randomized Clinical Trials (RCTs) as possible, which included controlled vocabulary and free text terms using field labels, Boolean, and proximity operators adapted for each search engine, without language restrictions, or other types of filters. The following electronic databases were searched: MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), from inception to April 2023. Additionally, we searched for clinical trials registries in the International Clinical Trials Registry Platform of the World Health Organization; and we hand-searched reference list of the selected studies. Search strategies are available in Supplementary Table 1.
Studies selectionTwo authors (CDB and LNB) independently reviewed the studies identified with the search strategy. Initially, they performed it by title and abstract, later by full text. Disagreements in the selection were resolved by consensus or by involving a third review author (MCV).
Eligibility criteriaType of studies included: RCTs with at least two comparison arms, available as a full publication. Studies published only as conference abstracts or posters were not considered.
Type of participants: HIV-positive adults, with CMV viremia, without evidence of EOD. Individuals with established antiviral treatment for pathologies other than CMV were excluded.
Types of interventions: Aciclovir, ganciclovir, valaciclovir, valganciclovir, foscarnet o cidofovir, independent of dose, route of administration, or scheme duration.
Type of comparator: No therapy or placebo administration.
Type of outcome measures: The primary outcomes evaluated was the incidence of CMV EOD and serious adverse events.
CMV EOD defined as the diagnosis of CMV infection in association with one or more of the following: retinitis, pneumonitis, focal gastrointestinal disease, impaired liver function, and/or encephalitis.
Adverse event (AE) defined as any adverse medical event associated with the use of a drug, whether or not considered drug related.
Serious adverse event (SAE) defined as any AE occurring at any dose that results in any of the following outcomes: 1) death; 2) life-threatening event; 3) hospitalization or extension of existing hospitalization; 4) persistent or significant disability or a substantial interruption of the ability to perform normal life functions; 5) congenital birth defect.
The secondary outcomes evaluated were: 1) death from all causes, 2) death from CMV EOD, 3) non-serious adverse events, 4) withdrawal of treatment due to adverse events and 5)iIncidence of opportunistic diseases.
Risk of bias assessmentTwo authors (CDB and LNB) independently performed the risk of bias assessment for each included study, using the Cochrane risk-of-bias tool for systematic intervention reviews (RoB 1.0).10 The domains evaluated were 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and staff, 4) blinding of outcome assessor, 5) incomplete outcome data, 6) selective reporting, and 7) other biases. Disagreements were resolved by consensus or by involving a third review author (MCV).
Data extractionTwo review authors (CDB and LNB) independently performed the extraction of the following data in each of the included studies, using a data extraction form, previously designed and tested: location and year of the study, inclusion and exclusion criteria, baseline information of participants, characteristics of both the intervention and the comparator and, lastly, characteristics of the outcomes evaluated.
AnalysisAnalysis was carried out using Review Manager software (RevMan 5.3). For dichotomous outcomes, results are presented as OR with their 95 % CI, displayed in forest plot figures. Investigators of included studies were contacted to request missing data when necessary. The clinical heterogeneity was assessed by analyzing the variability in the studies, by differences in the characteristics of the participants, the interventions, the comparators, and the way of measuring the evaluated results. When analyzes of aggregated results were performed, statistical heterogeneity was assessed by visual inspection of the forest plot and using the I2 statistical test, considering heterogeneity that may not be important from 0 to 40 %; moderate from 30 to 60 %; substantial from 50 to 90 %; and considerable from 75 to 100 %. Regarding the quality of evidence, we used the GRADE approach, specifying four levels of quality (high, moderate, low, and very low), taking into account the following factors: risk of bias, inconsistency of the results, indirect evidence, imprecision, and publication bias.11
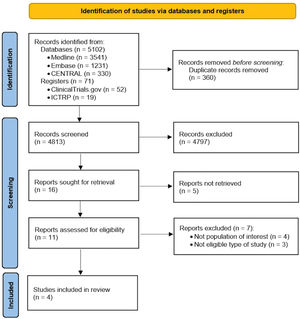
ResultsSearch resultsA total of 5173 references were identified from the electronic search in databases and other mentioned sources. After removing duplicates and performing an initial screening by title and abstract, 16 references were eligible for full-text evaluation. Of these, a total of 12 studies were excluded: five because the full text was not available despite contacting the authors, four because the types of participants of interest were not included (inclusion of participants with HIV and positive CMV immunoglobulin G serology or culture instead of CMV viremia), and three because they were not ECAs. A total of four studies were included in the present systematic review 12–15 as shown in the PRISMA flow diagram (Fig. 1). The excluded studies and the reason for exclusion are found in Supplementary Table 2.
Included studiesFour RCTs were included, for a total of 397 participants.12–15 Three studies were conducted in USA 12–14 and one study in France.15 98 % were male, with a mean age for men and women of 40.5 years. The baseline mean CD4 cell count was 20.2 cells/mL and the baseline CMV viral load ranged from 400 to 2 300 000 copies/mL. 79 % to 100 % were on HAART.12,14 See Table 1.
Characteristics of the studies included in the analysis.
| Study | Country | Study design | Inclusion criteria | Exclusion criteria | Intervention | Comparator | Outcomes | Authors’ conclusions |
|---|---|---|---|---|---|---|---|---|
| Balfour (1996) | The United States | Multicenter, open-label, randomized clinical trial | Patients with AIDS, age >18 years, Karnofsky ≥ 60, with life expectancy ≥ 6 months, CD4 counts less than 200 mL, CMV viremia, who have never manifested invasive CMV disease (n = 27). | Pregnant or lactating women, active CMV disease, previous treatment of CMV disease with foscarnet or ganciclovir, treatment with acyclovir in recent weeks, and treatment with renal tubular excretion inhibitors or loop diuretics. | Foscarnet in four different doses, administered IV for 10 days: 15 mg/kg every 8 h, 30 mg/kg every 8 h, 45 mg/kg every 12 h, 90 mg/kg every 12 h (n = 22). | Non- treatment (n = 5) | CMV EODa, all-cause mortalitya | Reductions in the levels of CMV and HIV-1 viremia correlated quantitatively with systemic exposure to foscarnet, whereas control subjects experienced an increase in CMV and HIV-1 burdens. |
| Salmon-Ceron (1999) | France | Multicenter, open-label, randomized clinical trial. | Age > 18 years, with CD4 cell count ≤ 100 mL, two positive blood cultures for CMV in the three months prior to inclusion (the last within 14 days prior to inclusion), no active or past CMV organ disease (n = 42). | Unexplained fever or other symptoms suggestive of CMV target organ disease or any of the following: a hemoglobin level less than 9 g/dL, serum creatinine > 150 mmoL/L, or serum calcium or phosphate ≥ 20 % above or below normal. | Foscarnet 100 mg/kg every 12 h IV for 14 days (n = 21). | Non- treatment (n = 21) | CMV EODb, all-cause mortalitya, adverse eventsa, withdrawal of the therapya and withdrawal of the therapy due to adverse eventsa | Sequential courses of intravenous foscarnet might not be a good strategy for preemptive therapy in this population. In patients with a positive blood marker, treatment able to induce and maintain negative CMV blood cultures could constitute an effective intervention. |
| Spector (1998) | The United States | Randomized, double-blind, clinical trial. | HIV-positive adults with CD4 ≤ 50 cells/mL on two occasions within 30 days prior to randomization or CD4 count ≤ 100 cells/mL in those with documented history of AIDS-defining opportunistic infections and baseline CMV-positive viral load (n = 281). | Past or present CMV disease, history of treatment for CMV, active gastrointestinal disease, absolute neutrophil count < 750 cells/mL, platelet count < 50,000 cells/mL, estimated creatinine clearance rate < 70 mL/min or a score < 60 on the Karnofsky scale | Ganciclovir 1000 mg orally every 8 h, mean duration 269 days (n = 191). | Placebo (n = 90) | CMV EODc | In persons with advanced AIDS, phophylactic oral ganciclovir significantly reduces the risk of CMV disease. |
| Wohl (2009) | The United States | Randomized, double-blind, clinical trial. | Adults with HIV, with IgG (+) for CMV, no evidence of EOD, with CD4+ < 100 mL and plasma HIV CV > 400 copies/mL in the 30 days prior to admission. Subjects had to have been receiving ART continuously for three months or not receiving and not planning to start ART (n = 47). | Not defined by the authors | Induction with valganciclovir 900 mg/kg orally twice daily, followed by maintenance therapy with valganciclovir 900 mg/kg orally every day, mean duration 54.7 weeks (n = 24). | Placebo (n = 23) | CMV EODd, all-cause mortalitya | Preemptive anti-CMV therapy in patients with persistently low CD4+ cell counts in the current treatment era may not be warranted given the low incidence of CMV EOD and high all-cause mortality observed in this study population. |
CMV retinitis was defined by its characteristic funduscopic appearance and confirmed by photographs and angiography in doubtful cases. CMV disease at other sites was defined by the combination of suggestive clinical symptoms, macroscopic lesions, histologic evidence of CMV intranuclear inclusions, and/or positive culture for CMV.
The presence of CMV retinitis was determined through examination of the fundus of the dilated eye and through indirect ophthalmoscopy by ophthalmologists experienced in the diagnosis of the condition. A diagnosis of CMV gastrointestinal disease was confirmed by the presence of signs and symptoms of disease in the upper or lower gastrointestinal tract and by endoscopy with biopsy. The biopsy had to reveal the presence of cells with CMV inclusions or evidence of CMV on immunostaining, immunofluorescence, or in situ hybridization; inflammation or necrosis; and the absence of other pathogens. A diagnosis of CMV pneumonia required confirmation by either open-lung biopsy or transbronchial lung biopsy. The lung biopsy had to reveal cells with CMV inclusions, or the tissue had to test positive for CMV on immunostaining, immunofluorescence, or in situ hybridization; in addition, there had to be no evidence of P. carinii or other pathogens. At least two of the following were also required for a confirmed diagnosis of CMV pneumonia: interstitial infiltrates seen on chest x-ray films, dyspnea, a need for supplemental oxygen or ventilatory assistance, and decreased partial pressure of oxygen. The diagnosis of CMV polyradiculopathy required confirmation of CMV in the cerebrospinal fluid by culture or the polymerase chain reaction, as well as progressive flaccid paraparesis, polymorphonuclear pleocytosis, and decreased glucose levels and elevated protein in the cerebrospinal fluid. Other types of CMV disease required confirmation by biopsy and had to fulfill the histopathological and virologic criteria described above.
Confirmed CMV retinitis: Typical lesions including white areas with or without hemorrhages and/or gray-white areas of retinal necrosis with or without hemorrhages. Lesion(s) has/have irregular, dry-appearing, granular border, with little or no overlying vitreous inflammation. Must be diagnosed by an experienced ophthalmologist using indirect ophthalmoscopy and documented by retinal photography that can be independently verified; confirmed CMV esophagitis: Presence of at least one of the following symptoms: retrosternal pain or odynophagia (pain on swallowing) AND appropriate visualization procedure (endoscopy) that reveals mucosal erythema, erosion, or ulceration AND tissue biopsy demonstrating CMV by antigen or characteristic cytopathic changes; confirmed CMV gastroenteritis: Presence of abdominal pain AND appropriate visualization procedure (endoscopy) that reveals mucosal erythema, erosion, or ulceration AND tissue biopsy demonstrating CMV by antigen or characteristic cytopathic changes; confirmed CMV colitis: Presence of at least one of the following symptoms: abdominal pain or diarrhea (typically in small volume and associated with mucus and blood) AND appropriate visualization procedure (colonoscopy, sigmoidoscopy, or endoscopy) that reveals mucosal erythema, erosion, or ulceration AND tissue biopsy demonstrating CMV by antigen or characteristic cytopathic changes; confirmed CMV proctitis: Presence of rectal pain, often associated with tenesmus, mucus, and blood AND appropriate visualization procedure (colonoscopy, sigmoidoscopy, or proctoscopy) that reveals mucosal erythema, erosion, or ulceration AND tissue biopsy demonstrating CMV by antigen or characteristic cytopathic changes; confirmed CMV pneumonitis: Hypoxemia and infiltrates on chest X-Ray or CT/MRI scan. AND tissue biopsy or cells obtained by BAL demonstrating CMV by antigen or characteristic cytopathic changes AND no other pathogens identified by routine testing (see instructions) OR signs/symptoms persist or recur after treatment of copathogens; confirmed CMV encephalitis: Progressive change in mental status, delirium, rapidly progressive cognitive impairment, or signs and symptoms of brain stem injury AND detection of viral nucleic acids (e.g., PCR) in CSF or CSF CMV culture positive or brain biopsy demonstrating CMV by antigen, detection of viral nucleic acids (e.g., PCR), or characteristic cytopathic changes; confirmed other CMV syndromes: Hepatitis or cholangitis: ALP or ALT significantly elevated above the patient's baseline values AND tissue biopsy demonstrating CMV by antigen or characteristic cytopathic changes. Radiculomyelopathy: Clinical presentation compatible with CMV EOD, including all of the following: a. Decreased lower extremity strength and reflexes or syndrome consistent with a cord lesion presently subacutely (over days to weeks); b. Myelogram or MRI reveals no mass lesions but lower spinal nerve roots thickened; c. CMV-positive culture in CSF OR detection of CMV viral nucleic acids (e.g., PCR) in CSF; Confirmed cutaneous CMV ulcers: Direct visualization of oral or vulvovaginal or perianal ulcers AND CMV culture of lesion or histologic demonstration of typical CMV cytopathology on biopsy of lesion.
Foscarnet was the therapy of choice in two of the four included studies,12,15 although the dose, frequency and duration of the drug varied between studies. In the other two studies, the antiviral evaluated was ganciclovir13 and valganciclovir.14 Regarding the characteristics of the comparator, in half of the studies it was placebo,13,14 while in the other two studies it was no treatment.12,15
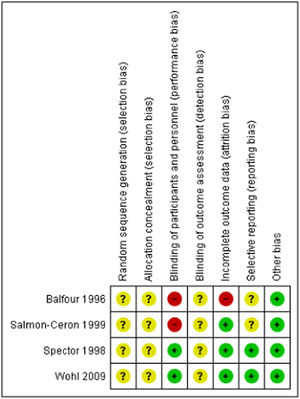
Risk of biasFig. 2 and Supplementary Fig. 1 summarize the ‘Risk of bias’ assessment for each of the included studies. For the domains of random sequence generation, allocation concealment and blinding of outcome assessment, the four trials did not report their methods in sufficient detail to permit judgement and therefore we classified were at unclear risk of bias. The blinding of participants, although two of the studies were open-label studies, were considered as objectively measured and we considered that the participants' knowledge of the allocation group was unlikely to alter the results seriously. Details are provided in Supplementary Table 3.
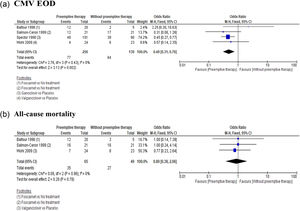
Primary and secondary outcomesCMV EOD: This outcome was evaluated in all included studies. The pooled analysis evidenced a favorable effect with the use of preemptive therapy compared to placebo or non-therapy, with an estimated OR = 0.49 (95 % CI 0.31‒0.76), (Fig. 3a). The quality of the evidence was low due to the limitations of unclear risk of bias, and imprecision due to small sample sizes and few trials.
All-cause mortality: This outcome was evaluated in three of the included studies12,14,15 without finding a statistically significant difference in any of the cases, with a pooled OR = 0.89 (95 % CI 0.38‒2.06), (Fig. 3b). The quality of evidence was low due to limitations of unclear risk of bias, and imprecision due to small sample sizes and few trials.
Adverse events (AE): The outcome of adverse events was evaluated in one study,15 with three patients experienced minor adverse events at days 3, 6, and 10 of foscarnet therapy (genital ulcers in two and hypocalcemia in one patient), and no events in the non-treatment group; OR = 8.13, (95 % CI 0.39‒167.98). The quality of evidence was low due to limitations of unclear risk of bias, and imprecision due to small sample size in only one trial.
Serious adverse events (SAE): This outcome was not evaluated or reported in any of the included studies.
Withdrawal of treatment due to adverse events: This outcome was evaluated in one study,15 that compared foscarnet versus non-treatment. In this study, three patients in the antiviral therapy group discontinued the treatment: two because of genital ulcers and one secondary to grade 1 hypocalcemia; OR = 8.13 (95 % CI 0.39–167.98). The quality of evidence was low due to limitations of unclear risk of bias, and imprecision due to small sample size in only one trial.
Mortality associated with CMV EOD and the incidence of other opportunistic diseases were not assessed in any of the studies included in the analysis.
DiscussionWe identified four clinical trials that evaluated the efficacy and safety of different schemes of preemptive therapy in individuals with advanced HIV and CMV viremia. In the included studies, we found a lower probability of developing CMV EOD, but we did not find a difference in all-cause mortality with the use of preemptive therapy compared to placebo or no treatment.
In contrast to our findings, a recent systematic review16 did not find a reduction in the incidence of CMV EOD (RR = 0.84, 95 % CI 0.59‒1.18), but did found a reduction in the Relative Risk (RR) of all-cause mortality rate (RR = 0.85, 95 % CI 0.74‒0.97). This discrepancies in the results may have been due to differences in the types of participants included in each review. Our eligibility criteria are stricter, as we included only patients with HIV and confirmation of CMV viremia and excluded positive culture or CMV serology (ELISA or radioimmunoassay). The reason for the exclusion of these patients was the high CMV seroprevalence reported worldwide, reaching values of 90 % in developing countries.1,2 This may lead to reduction of potential selection biases that could affect the results found.
Our findings are of greater importance, especially in developing countries, where a delay of more than four weeks between HIV diagnosis and initiation of HAART has been reported, leading to an increase in the incidence of AIDS-defining events.17,18 Regarding the safety profile of preemptive therapy, no SAEs were reported in any of the included studies; only one study13 reported AEs related to foscarnet use, but this finding was not statistically significant.
Our results are in line with retrospective observational studies in HIV-positive people and CMV viremia. Mizushima et al.19 reported a decrease in incidence density from 230 cases per 1000 person-years to 60.9 cases per 1000 person-years in the preemptive therapy group, with an estimated HR = 0.286 (95 % CI 0.087‒0.939). However, the applicability of our result to areas with high and prompt antiretroviral use might be limited because of the low number of patients with EOD, as shown in a study from Spain.20
Several studies have shown that the presence of CMV viremia may be an independent predictor of mortality, even after adjusting for HIV viral load level or CD4 cell count.21–23 Despite this, the studies included in the present review did not demonstrate significant differences between preemptive therapy and placebo or non-treatment for the outcome of all-cause mortality, suggesting that other factors different to CMV infection might be responsible for the fatal outcome. Similarly, observational studies have not demonstrated a favorable effect of preemptive therapy for mortality, most of them with small sample sizes and methodological limitations due to their retrospective nature.19,24 Considering the aforementioned findings, it is not possible to draw conclusions about the real usefulness of preemptive therapy for the reduction of all-cause mortality in patients with HIV and CMV viremia.
The current version of the guidelines for prevention and treatment of opportunistic infections in adults and adolescents with HIV 25 does not recommend the use of preemptive therapy against CMV EOD. This recommendation is based on the study by Wohl et al.14 which failed to demonstrate a significant difference between the use of valganciclovir and placebo for the incidence of CMV EOD. However, several methodological limitations of this study should be taken into account: first, the number of patients included was 22 % lower than the expected sample size, which directly affects the statistical power of the study; secondly, the overall risk of bias was moderate because it did not provide information about the randomization method used, whether or not allocation concealment was performed or whether there was masking of the evaluators of the results. Based on the above, we consider that the available evidence is insufficient to determine the lack of benefit of preemptive therapy for preventing CMV EOD. It is likely that the results supporting the guideline recommendation are not generalizable to developing nations, where prompt antiretroviral treatment is not often feasible.
Our systematic review has several limitations. First, despite an exhaustive electronic search, the total number of trials included was relatively low, and their sample sizes small. It is likely that the limited number of studies included in our analysis is result of the advent of HAART. Second, the unclear risk of bias for the domains of random sequence generation, allocation concealment and blinding of outcome assessment; both aspects which influenced the low quality of evidence obtained for the outcomes assessed. Third, the most recent included RCT was published over a decade ago (in 2009), and three of the four included studies were published in the 90 s, a time when the incidence of AIDS and CMV infection was higher than today, and HAART was not widely used. Besides, two of the four studies have used foscarnet as therapy, a less preferred option due to its pharmacokinetic and safety profile, and the one study made with valganciclovir had a low sample.
ConclusionsOur systematic review provides relevant information about the use of preemptive therapy in people living with HIV and CMV viremia. We found a benefit of this therapy for the prevention of CMV EOD in this population, with acceptable safety profile. Considering the risk of bias and imprecision of the included studies, the decision about the use of preemptive therapy in these patients should be taken with caution and individualized. RCTs or observational studies with methodological rigor are needed to further investigate the utility of this therapy, as well as the selection of the best drug regimen its duration.
Authors’ contributionsCDB wrote the first protocol. MCV carried out the search strategy and contributed to the methodological advice, risk of bias assessment, analysis of results. CDB and LNB carried out the studies selection, data extraction, risk of bias assessment, writing of the results and discussion sections. JAC contributed to the methodological advice, writing of the introduction and discussion sections.
Ethical disclosureProtection of human and animal subjects. This research does not use animal nor human material or clinical data from patients.
Funding statementNo direct financing sources were received.