Epidermal parasitic skin diseases encompass scabies, pediculosis, cutaneous larva migrans, myiasis, and tungiasis. Tungiasis is probably the most neglected of all Neglected Tropical Diseases (NTD). It occurs in South America, the Caribbean and Sub-Saharan Africa and affects marginalized populations where people live in extreme poverty. In endemic communities the prevalence can be up to 30% in general population and 85% in children. Over time, chronic pathology develops characterized by hyperkeratosis, edema around the nail rim, fissures, ulcers, deformation and loss of nails. This leads to a pattern of disabilities, eventually resulting in impairment of mobility.
Dimeticones are a family of silicon oils with a potential to kill parasites located on top or inside the epidermis by a physical mode of action. They are considered the treatment of choice for pediculosis capitis and pediculosis pubis. With regard to tungiasis, the so called rear abdominal cone of the parasites has been identified as a target for treatment with dimeticones. NYDA®, a mixture of two dimeticones with different viscosity, is the only dimeticone product for which data on the mode of action, efficacy and safety with regard to tungiasis exists. The product has been shown highly effective against embedded sand fleas, even in very intense infection with more than 500 parasites situated on top of each other. A randomized controlled trial showed that seven days after a targeted application of NYDA® 97% (95% CI 94–99%) of the embedded sand fleas had lost all signs of viability.
Comprehensive toxicological investigations on the dimeticones contained in NYDA® showed that there is practically no risk of embryotoxicity, fetotoxicity, teratogenicity, and other toxicity. The safety of dimeticones was also demonstrated in clinical trials with a total of 106 participants with tungiasis, in which not a single adverse event was observed.
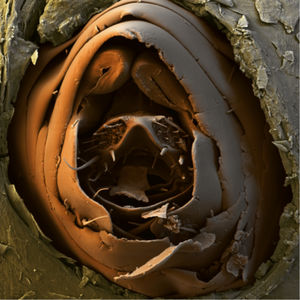
Tungiasis (sand flea disease) is a parasitic skin disease with origins in the Americas. A multitude of local designations indicate that tungiasis originally occurred in most countries and populations of the Americas.1 In some areas tungiasis is still so common that it became part of the local folklore (Fig. 1).
Data on geographical disease occurrence in the Americas is scanty, but indicate that tungiasis exists in Mexico, Honduras,2 Venezuela, Colombia,3 Ecuador, Peru, Paraguay, Argentina, Haiti, Barbados, and Trinidad.3,4 In Brazil tungiasis is reported from Amazonia State in the North to Rio Grande do Sul State in the South.5 Several community-based studies performed in Brazil, Haiti and Trinidad-Tobago showed astoundingly high prevalence in resource-poor rural and urban communities. Prevalence ranged from 10.6 to 82.6% in the general population.
Tungiasis seems to be frequent in Amerindian populations in the Amazon/Orinoco lowlands of Colombia and Venezuela. In Amerindian populations, tungiasis is associated with severe morbidity potentially leading to death (Heukelbach, unpublished observation, 2012; Grundmann, unpublished observations, 2010; Roger, unpublished observation, 2017). Even in children ≤1 year the prevalence was 2%.3
How intense transmission can be in a resource-poor setting is demonstrated by a study in a favela in Fortaleza, Northeast Brazil. All individuals who entered the community after being out of the endemic area for some time became infected within three weeks. In this setting the average attack rate was 15 fleas per person per week.6
Usually, transmission occurs peri-domiciliary. When houses do not have a solid floor, and domestic animals can roam in an out, intra-domiciliary transmission is frequent.7–9 In Amerindian communities, commonly the whole lifecycle of the parasite is completed inside the house.10
Morbidity is triggered by an intense inflammatory response around embedded female sand fleas11 leading to pain and intense itching. Bacterial super-infection of lesions is almost constant and intensifies the inflammation.12 In non-immunized individuals, tetanus is a known fatal complication.13 Over time, with constant re-infection, chronic pathology develops characterized by hyperkeratosis, edema around the nail rim, fissures, ulcers, deformation and loss of nails. This leads to a pattern of disabilities finally resulting in partial or total immobility.14 Children, persons with disabilities and the elderly bear the highest disease burden.11,15
The aim of this review is to critically evaluate the efficacy and safety of dimeticones in the treatment of tungiasis.
MethodologyStudy designInitial searches revealed that published studies on the treatment of tungiasis varied considerably in terms of intervention types, outcome measures, duration of treatment and length of follow-up, making a meta-analysis or a systematic review unfeasible. Hence, a narrative-style review of publications, using systematic data searches, was performed.
Search strategiesStudies evaluating the efficacy of treatments against tungiasis were collected from a set of databases using the specific search terms ‘tungiasis’, ‘Tunga*’, ‘Tunga penetrans’, ‘sand flea disease’, ‘efficacy’ and ‘safety’. The databases searched included MEDLINE, PubMed, SciELO and Lilacs. Databases were searched from inception to 31 December 2018.
To identify studies not indexed in the databases listed above, grey literature was searched in OpenGrey and PQDT Global (ProQuest Dissertations & Theses Global).
Study selectionDue to the small number of publications, all studies investigating the efficacy and safety of dimeticones in the treatment of epidermal parasitic skin diseases were included, regardless of study population, intervention type, outcome measures, duration of follow-up, and language.
ResultsVarious insecticides and anti-helminthics have been tested regarding their activity against embedded sand fleas, using systemic or topical application. In a randomized controlled trial, the efficacy of the topical application of ivermectin lotion (0.8% w/v), metrifonate (trichlorfon) lotion (0.2% w/v), thiabendazole lotion (0.2% w/v), thiabendazole ointment (5% w/v) were compared to placebo lotion.16 The study showed a light effect of ivermectin lotion, metrifonate lotion and thiabendazole lotion. A randomized controlled study with oral ivermectin did not show any effect at all.17
Hitherto, only for one commercially available dimeticone product data on mode of action, efficacy against ectoparasites and safety is available. NYDA® (Pohl-Boskamp GmbH & Co. KG, Hohenlockstedt, Germany) contains a mixture of two dimeticones differing in viscosity and volatility, which account for the special creeping and spreading properties of NYDA®. The high- and the low-viscous dimeticones are the functional components responsible for the physical mode of action.18,19 NYDA® is a certified medical device according to Medical Device Directive in Europe. It is registered for the treatment of pediculosis capitis in over 30 countries worldwide.
NYDA® enters openings as small as 10μm and rapidly covers microscopic surfaces.20 The dimeticone with the low viscosity evaporates and the dimeticone with the high viscosity forms an oil film which closes the openings and seals microscopic surfaces.
NYDA® also contains medium-chain triglycerides and jojoba oil. The medium-chain triglycerides and the jojoba oil are added as hair and skin nourishing components and to increase the cosmetic performance of the formula. They do not have an effect on ectoparasites.18,19 NYDA® does not contain any component with a neurotoxic mode of action.
Mode of action of dimeticonesDimeticones (synonyms: polydimethylsiloxanes; silicone oils) consist of fully methylated linear siloxane polymers containing repeating units of the formula [(CH3)2SiO] with trimethylsiloxy end-blocking units of the formula.21 They are translucent, almost odorless fluids.22
The mode of action of dimeticones was investigated in head lice (Pediculus humanus capitis) and house crickets (Acheta domestica). When NYDA® was applied on the abdomen and thorax of the insects, the dimeticone rapidly spread over the chitinous cuticle of the insects, producing a closed oil layer, and within 1min, entered the respiratory tract through the 14 stigmata (the entries to the tracheal system, located at the lateral side of the insect).20
In house crickets, the insect's head trachea was completely filled with NYDA® within less than 1min; in head lice the filling of the entire tracheal system occurred within 3.5min.20 This resulted in blocking the oxygen supply to the brain.
Since the cuticle of the blood-sucking insects becomes completely covered with a film of the high-viscous dimeticone, they are unable to eliminate the water they have taken up with a blood meal, eventually leading to disruption of the intestine.18 Either mode of action is purely physical. Similar observations were made in pig lice, parasites that are much larger than human lice17 (Mutebi F and Feldmeier H, unpublished observation).
It is difficult to envisage how an insect can develop resistance against a compound which has a purely physical mode of action, particularly if the parasites are killed within a very short time.
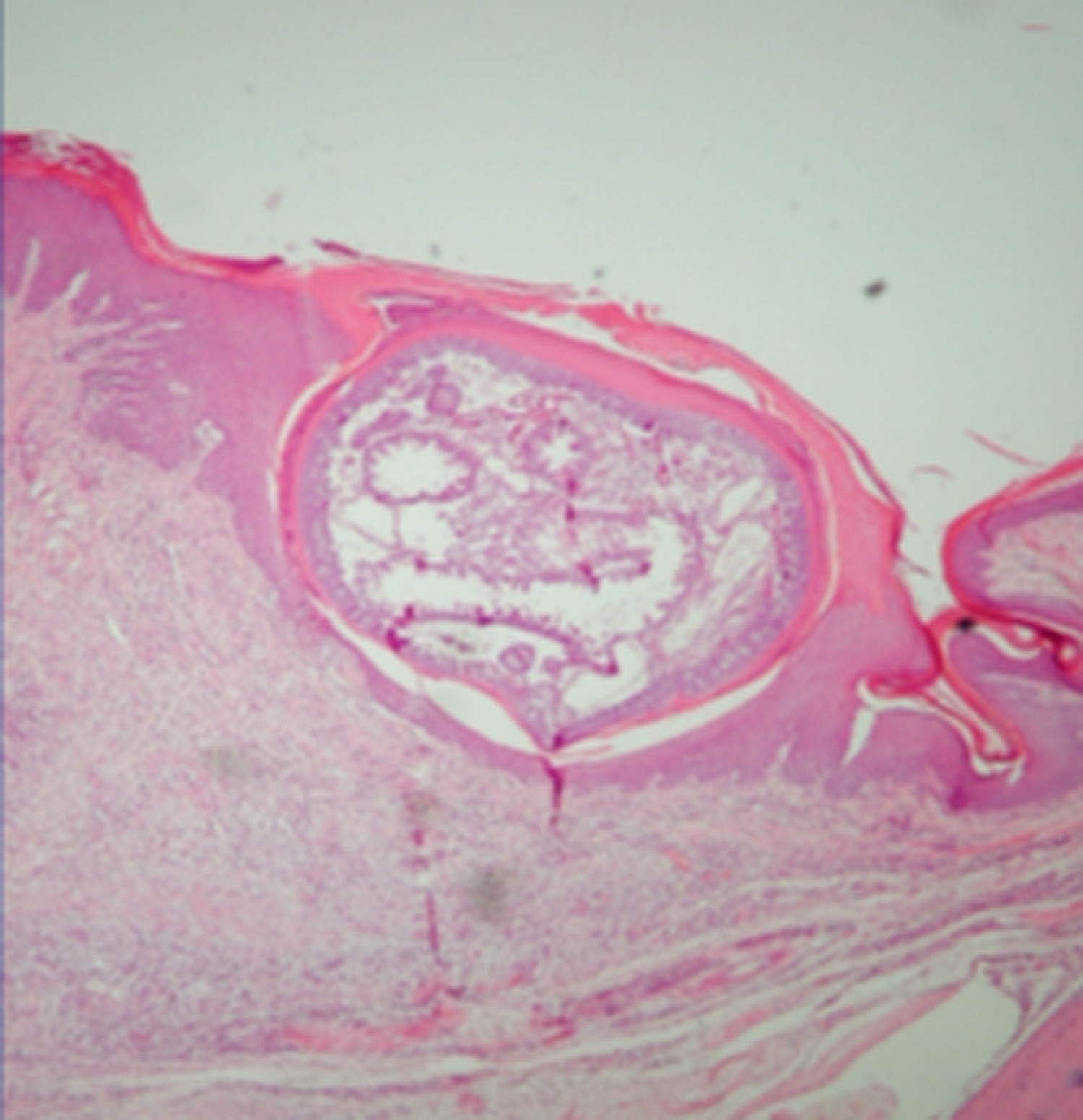
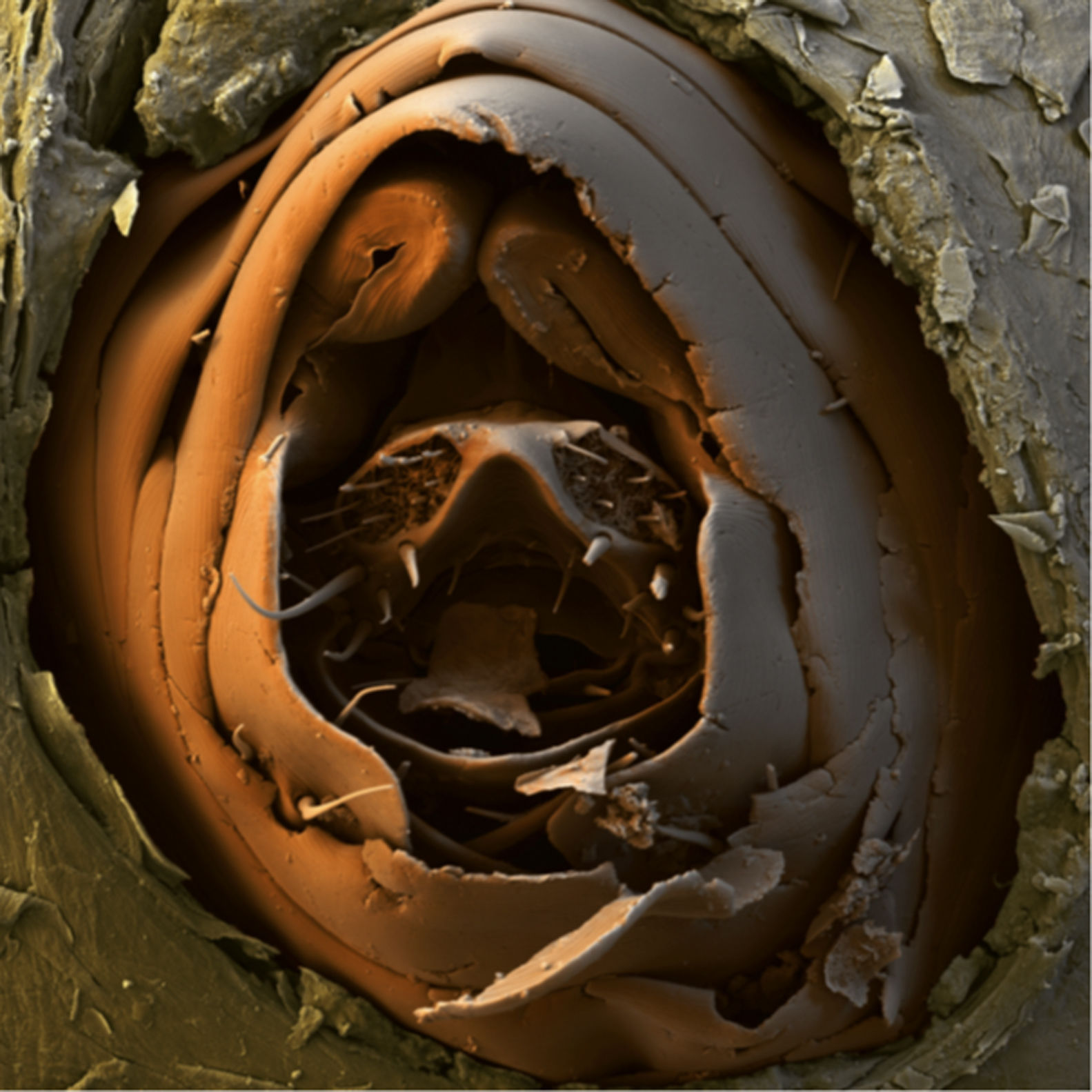
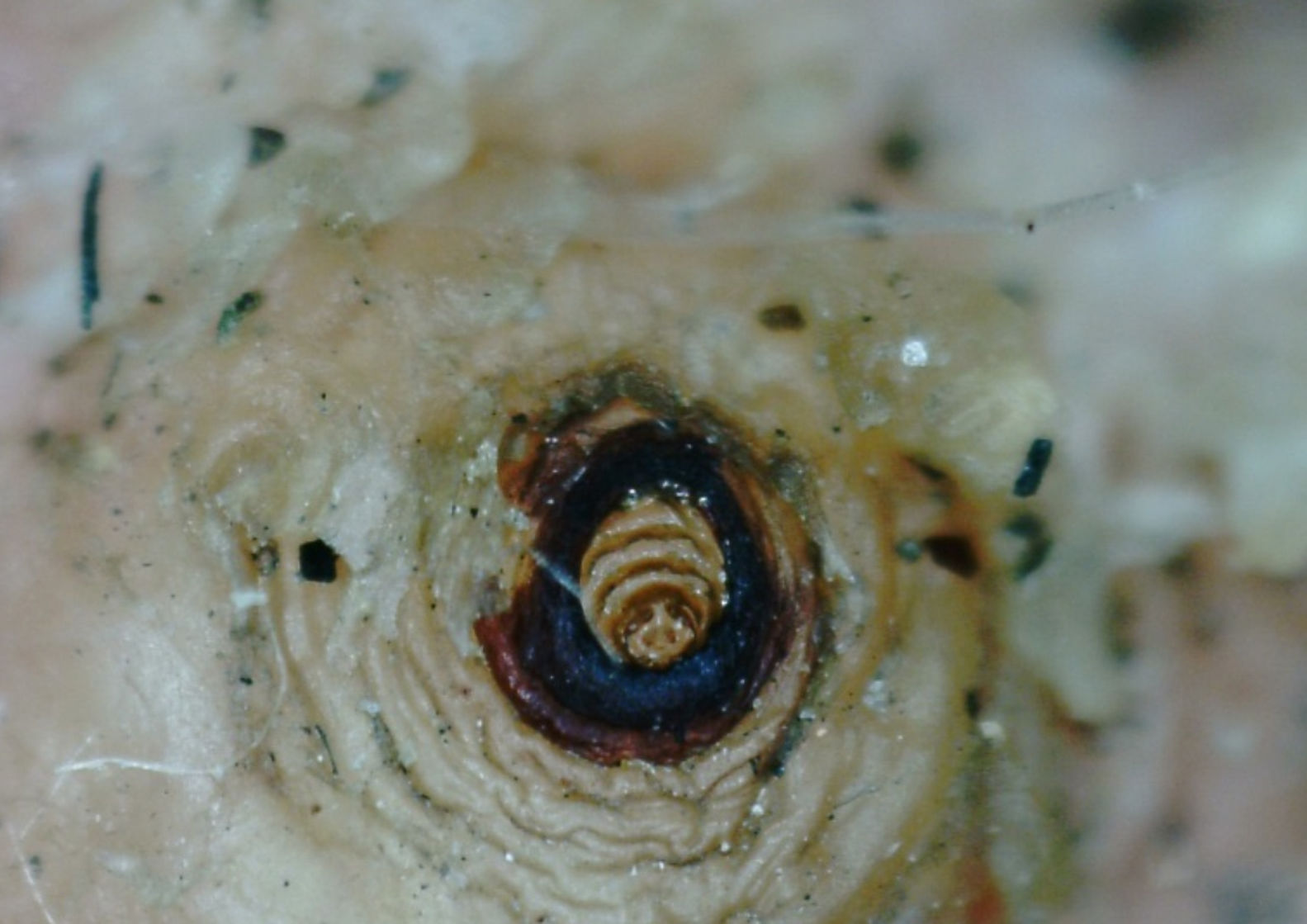
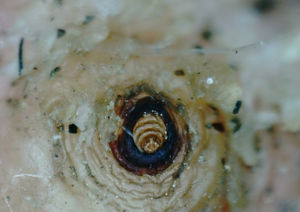
Rationale for the use of dimeticones in tungiasisOnce a female sand flea has become embedded in the skin (Fig. 2), it remains in contact with the environment through a tiny opening in the corneal layer (Figs. 3 and 4). In the so-called rear abdominal cone which protrudes above the skin, a number of vital organs begin/end, namely the respiratory tract, the intestinal tract and the genital tract. The diameter of the openings of the respective tracts is not precisely known but is considerably less than 20μm. Only fluids with no surface tension can penetrate into such small openings.
To confirm the physical mode of action of NYDA® against embedded sand fleas, a study in naturally infected pigs was performed in Uganda (Mutebi F and Feldmeier H, unpublished observation). Five drops were targeted to the rear abdominal cone of an embedded sand flea. The embedded sand fleas were removed surgically and were examined using a handheld light microscope to establish if the dimeticone has crept into the rear abdominal cone. In all cases the abdominal cone of embedded sand fleas was sealed by the high viscous dimeticone and the physical mode of action of dimeticones on embedded sand fleas was confirmed (Mutebi and Feldmeier, unpublished observation).
Efficacy of dimeticonesEfficacy in the treatment of pediculosisA randomized, controlled, observer-blinded clinical trial was performed in a poor neighborhood in Brazil.23 Children aged 5–15 years with a high intensity of infestation with head lice were treated twice with NYDA® seven days apart. NYDA® was compared to permethrin 1%, the local standard treatment. The scalp and the hair were wetted with NYDA® according to the instructions of the producer. After 8h the hair was washed with a commercial shampoo not containing dimeticones. A normal comb was used to spread the liquids evenly. Overall cure rates were: day 2 (after first treatment) – dimeticone 94.5% (95% CI: 86.6–98.5%) and permethrin 66.7% (95% CI: 54.6–77.3%; p<0.0001); day 9 (after second treatment) – dimeticone 97.2% (95% CI: 90.3–99.7%) [p<0.0001] and permethrin 67.6% (95% CI: 55.4–78.2%) [p<0.0001].
In two in vitro studies the efficacy of NYDA® on killing head lice embryos was investigated using a Hair Tuft Bioassay. After an immersion of viable head lice eggs for 30min in NYDA®, no eggs hatched. If the application time was reduced to 10min, 5–8% of the embryos survived and developed into adults. When 9–11 days old head lice eggs were treated with NYDA®, the efficacy of killing lice embryos was reduced to 97%.24,25
Anecdotal observations suggest that pubic lice are also highly susceptible to treatment with NYDA®.26
Efficacy in the treatment of tungiasisThe efficacy of NYDA® in the treatment of tungiasis was investigated in two randomized controlled studies performed in Kenya and Uganda, and in a case series in patients with extremely severe tungiasis in Colombia.
The topical application of NYDA® to the whole foot for 10min was compared to bathing the foot in KMnO4 for 10min with subsequent application of petroleum jelly. The latter is the standard treatment recommended by the MoH of Kenya.15 Patients were monitored every second day for a total of 7 days. Outcome measures were disappearance of viability signs (expulsion of eggs, excretion of fecal thread, excretion of fecal liquid or pulsations/contractions of the parasite) assessed through a digital handheld microscope, abnormal development regarding the life stages defined in the Fortaleza classification11 and assessment of a local inflammation score.
Seven days after treatment, in the dimeticone group 78% (95% CI 67–86%) of the parasites had lost all signs of viability compared to 39% (95% CI 28–52%) in the KMnO4-petroleum jelly group (p<0.001). In the dimeticone group 90% (95% CI 80–95%) of the penetrated sand fleas showed an abnormal development already after five days, compared to 53% (95% CI 40–66%; p<0.001) in the KMnO4-petroleum jelly group. Seven days after treatment, signs of local inflammation had significantly decreased in the dimeticone group (p<0.001), but not in the KMnO4-petroleum jelly group. The little effect observed after bathing the foot in KMnO4 was attributed to the petroleum jelly which, in a tropical environment, becomes liquid and may have partially sealed the abdominal cone when KMnO4 had died.
The finding that the local inflammation rapidly disappeared when the parasite had died in situ showed that inflammation is causally related to the presence of a viable parasite. In practice, this means that there is no need to surgically remove embedded parasites after treatment with NYDA®: the dead parasites are eliminated by natural skin repair mechanism without delay (H. Feldmeier, unpublished observation, 2016).
In a study in rural Uganda, the efficacy of two distinct modes of application of NYDA® were compared: one targeted application to the area where the parasite protrudes through the skin and one comprehensive application to the whole foot, identical to the one used in the proof-of-principle-study.26 Whole foot treatment meant that dimeticone was applied to the skin of the foot up to the ankle. Targeted treatment meant that dimeticone was aspired into a 5-mL syringe to which a flexible tube was mounted. Three drops were applied to the area where the parasite's rear abdominal cone protruded through the skin. One drop corresponded to approximately 50μL of dimeticone. This procedure was repeated three times within 10min to ensure that a maximum amount of dimeticone entered the rear abdominal cone of the parasite and sealed it.
At the first follow-up, two days after treatment, the number of viable parasites decreased significantly in both groups. The loss of viability was higher in the targeted treatment group (p<0.001). At the second follow-up, after five days, the loss of viability was still higher in the targeted treatment group compared to the whole foot treatment group (p<0.02). After seven days, the reduction of the number of viable sand fleas was similar in both groups: 95% (95% CI 92–99%) of the parasites in the whole foot treatment group had lost all signs of viability and 97% (95% CI 94–99%) in the targeted treatment group. The authors concluded that the targeted application killed embedded sand fleas more rapidly compared to when the whole foot was covered. Besides, substantially less dimeticone was needed.26
In a case series of patients with very severe tungiasis in Vaupés Department, Colombia, five patients being in a life-threatening condition were identified in Amerindian communities. All patients showed hundreds (up to a thousand) embedded sand fleas and presented ectopic localizations of embedded sand fleas such as at the ankles, the knees, the elbows, the fingers and around the anus. At the soles, embedded sand fleas were laying in multiple layers on top of each other.10 All affected body areas with embedded sand fleas were intensely wetted with NYDA® and treatment was repeated after 12–24h. All patients recovered rapidly. After three to four days, inflammation of the skin had regressed considerably, and patients could place their feet on the ground without feeling pain. After one week all lesions had developed into crusts and healing of the skin had started.10 No adverse events were observed.10
An important finding of the three studies was that the few parasites which remained viable did not expel eggs. This suggests that a systematic application of NYDA® on the community level may result in a total disruption of the off-host cycle – provided that there is no animal reservoir involved in maintenance of the cycle.
Taken together the three studies provide evidence that:
- •
NYDA® kills up to 97% of embedded sand fleas within 5 days;
- •
A targeted application acts more rapidly than wetting the whole foot and needs considerably less dimeticone. This makes the targeted application more cost-effective if the total number of lesions is <40 or if penetrated sand fleas are located in clusters;
- •
NYDA® is effective in infections with several hundred embedded sand fleas situated in excessive hyper-keratotic skin and located on top of each other. In these cases, the affected skin has to be wetted intensively and repeatedly with NYDA®.
During a study on scabies in indigenous communities in the Amazon lowland of Colombia, several individuals were identified having myiasis caused by Dermatobia hominis. The infection was successfully treated by the topical application of NYDA® (Hollman Miller, unpublished observation, 2018).
Safety of dimeticonesGeneral aspectsDimeticones are widely used in cosmetics and industrial food preparation. Dimeticones are used in urology, ophthalmology and dermatology.27 In the European Union (EU), high-viscous dimeticones are approved as antifoaming food additive ‘E 900’ at a maximum level of 10mg/kg in solid foodstuffs and 10mg/L in liquids. The acceptable daily intake is 1.5mg/kg of bodyweight.
NYDA® is a CE marked class IIa medical device registered for treatment of ectoparasites. NYDA® falls in EU regulatory class IIa according to Rule 4, Annex IX of the Council Directive 93/42/EEC of 14 June 1993 concerning medical devices, as amended by Directive 2007/47/EC. NYDA® is available on the market since 2006.19
High-viscous dimeticonesHigh-viscous dimeticones are of well-established use in humans regarding topical and oral administration (such as in cosmetics and drugs, particularly in drugs to treat flatulence) in doses exceeding larger than those applied in NYDA® for the treatment of pediculosis capitis.
In 2011, the European Centre for Ecotoxicology and Toxicology of Chemicals Joint Assessment of Commodity Chemicals (JACC)27 concluded that humans may be exposed to high-viscous dimeticones via oral or dermal contact without any health risk (IASON Consulting, 2016, unpublished document). In laboratory animals, the substances showed a low potential for absorption by these routes. Since dimeticones are metabolically inert, following ingestion, they are rapidly excreted unchanged in the feces.27
In rabbits, no teratogenic effects were found after implantation as well as after oral administration. None of the reproductive and developmental toxicity studies performed using rats and rabbits gave evidence of any adverse effect of high-viscous dimeticones on fertility, gestation, peri- or post-natal development after oral, dermal or implantation exposure.27
In summary, animal study data as well as clinical experience in humans showed that high-viscous dimeticones are highly unlikely to be absorbed at significant amounts by the human body irrespective of the route of application (dermal, oral, inhalation). The risk of embryotoxicity, fetotoxicity, teratogenicity, or any systemic toxicity in the fetus, breast-fed baby or children ≤2 years of age is judged as virtually non-existent (IASON Consulting, 2016, unpublished document).
Taken together, from the toxicological point of view, there are no objections to use high-viscous dimeticones for the topical treatment of ectoparasites in pregnant or lactating women as well as in children ≤2 years of age (IASON Consulting, 2016, unpublished document).
Low-viscous dimeticonesLow-viscous dimeticones are highly volatile substances that are potentially absorbed after inhalation but pass the skin only at low amounts. Animal toxicity data on the low-viscous dimeticones octamethyltrisiloxane, decamethyltetrasiloxane and hexadimethylsiloxane revealed no evidence of any detrimental effect on reproductive or developmental performance up to the highest doses tested after inhalation exposure (IASON Consulting, 2016, unpublished). As these low-viscous dimeticones are transferred into human breast milk only at trace amounts, the risk of adverse effects to the breast-fed baby can also be considered negligibly low. A toxicological review concluded that the risk of embryotoxicity, fetotoxicity, teratogenicity, or any systemic toxicity in the fetus, breast-fed baby or children ≤2 years of age can be judged as very low (IASON Consulting, 2016, unpublished document).
InflammabilitySince dimeticones contain oxygen, they are potentially inflammable. Their inflammability depends on the chain length of the molecule. Dimeticones with a short chain, have a low viscosity and a high volatility and therefore are inflammable at rather low temperatures. In contrast, dimeticones with medium to long chain are not volatile and need high temperatures to get ignited.
Experiments performed under standardized conditions showed that human hair impregnated with NYDA® cannot be easily ignited but needs a direct contact to a naked flame. Assuming that scalp hair covered with NYDA® might function as a wick enhancing the inflammability, and furthermore hair increases the surface in contact with oxygen, the risk to catch fire on human skin was considered very low. Nonetheless, it is recommended by the producer that after topical treatment with NYDA® direct contact to naked flames is avoided for 2h (Pohl-Boskamp, unpublished document, 2012).
Clinical studiesSafety of NYDA® in the treatment of head lice infestationA randomized controlled trial in children with head lice infestations showed that NYDA® can be considered as very safe. Except in two cases in which NYDA® accidentally came in touch with the eye and resulted in a temporary irritation of the conjunctiva, no adverse events occurred.23
Safety of NYDA® in the treatment of tungiasisIn contrast to ectoparasites such as lice and other siphonaptera species, sand fleas are embedded almost completely in the epidermis. To determine whether topically applied dimeticone can penetrate into the skin next to a lesion, a series of experiments was performed in naturally infected pigs in rural Uganda (Mutebi et al., 2018, unpublished). Five drops of NYDA® were applied on the rear abdominal cone of the embedded sand fleas on the digits of the pigs. After treatment, embedded sand fleas were surgically removed and were examined using a handheld light microscope to establish if the dimeticone had crept between the cuticle of the parasite and the host tissues. After treatment with NYDA® no dimeticone was detected on the outer surface of the embedded sand fleas and in the adjacent host tissue. The outer surface of the intersegmental skin remained whitish as it is normally the case.
In two randomized controlled trials with a total of 106 participants, not a single adverse event was observed, even when the whole foot was repeatedly covered with NYDA®.15,26 Moreover, patients liked the application of the dimeticone, because it softened rough and dry skin and gave it an appealing shine.15
DiscussionDimeticones are polymer silicone oils and have a pure physical mode of action on ectoparasites. Due to the physical mode of action development of resistance is highly unlikely. Although we do not restrict the documents analyzed by study population, type of intervention, outcome measures, duration of follow-up and language, we found a limited number of publications and gray literature on efficacy and safety of dimeticones for the treatment of parasitic skin diseases in our search. However, with the available evidence, we can conclude that dimeticones are very safe molecules and can be used topically even in small children and pregnant women. NYDA®, a mixture of two dimeticones with a different viscosity and volatility, has been shown to be effective in pediculosis capitis, pediculosis pubis, tungiasis, and myiasis.
Dimeticones are promising tools for the treatment of parasites residing on top or inside the skin, provided the dimeticone is able to target the Achilles heel of the respective parasite, such as the respiratory tract in lice and the rear abdominal cone in sand fleas.
Tungiasis, like other parasitic skin diseases, continues to affect the quality of life of many people, especially those from rural areas, with limited access to health services and low income. The development of research on prevalence, disease burden, complications associated with high infestation and bad therapeutic practices in the community, toxicity or adverse effects when dimeticones and NYDA are used on bleeding skin or skin that has lacerations as well as the development of new treatments and the evaluation of the efficacy and safety of those already available, is a way of contributing to the reduction of unfair inequalities in health that exist between these populations and the majority societies. Therefore, their study and dissemination of the results through scientific publications should be part of the research and public health agendas of the governments of endemic countries and collaborating centers.
Conflicts of interestThe authors declare no conflicts of interest.