The present study aimed to evaluate the effectiveness of two doses of CoronaVac in preventing SARS-CoV-2 symptomatic disease with virological confirmation, as well as in the prevention of COVID-19 moderate and severe cases. A test-negative unmatched case-control design was used, in which cases were patients with suspected COVID-19 (presenting at least two of the following symptoms: fever, chills, sore throat, headache, cough, runny nose, olfactory or taste disorders) with virological confirmation, and controls were those whose SARS-CoV-2 test was negative. As for exposure, participants were classified as unvaccinated, or vaccinated with a complete schedule. Suspected COVID-19 cases were identified from March to November 2021, in two cities located in the State of São Paulo, Brazil. All participants signed the Informed Consent Form before enrollment. RT-PCR results and vaccination data were obtained from the local surveillance systems. Up to two phone calls were made to obtain information on the outcome of the cases. A total of 2981 potential participants were screened for eligibility, of which 2163 were included, being 493 cases and 1670 controls. Vaccination, age, the reported contact with a COVID-19 suspected or confirmed case in the 14 days before symptoms onset, and the educational level were the variables independently associated with the outcome. The adjusted vaccine effectiveness for symptomatic COVID-19 (AVE) was 39.0 % (95 % CI 6.0–60.0 %). The AVE in the prevention of moderate and severe disease was 91.0 % (95 % CI 76.0–97.0 %). Our results were influenced by the waning of the Gamma variant, in the second trimester of 2021, followed by the increase in vaccination coverage, and a drop in the number of cases in the second half of the year. The study demonstrated the high effectiveness of CoronaVac in preventing moderate/severe COVID-19 cases.
The incorporation of vaccines into the arsenal of control measures, at the end of 2020, changed the course of the COVID-19 pandemic. A reduction in the incidence of the disease was observed, particularly in severe cases, hospitalizations and deaths.
An unprecedented effort in history by the academic community, research funding agencies, health care services, governments and the pharmaceutical industry, enabled the short-term development of safe and effective vaccines against SARS-CoV-2. The consultation of the WHO SARS-CoV-2 vaccine landscape demonstrates the strength of this effort. Almost 400 vaccine candidates were in different stages of development as of March 2023, of which 180 had reached the clinical investigation phase, and more than thirty were already on the market.1 Several platforms have been used in their development and manufacturing, some new, such as mRNA vaccines, viral vector vaccines, and protein subunit vaccines, as well as other technologies already widely used in vaccines’ manufacturing for other infectious agents, such as inactivated vaccines.
CoronaVac, an adjuvanted inactivated virus vaccine, developed by the pharmaceutical company SINOVAC, in partnership with the Butantan Institute, was the first vaccine available in Brazil. Phase 3 trials demonstrated its safety, and an efficacy in preventing symptomatic disease caused by SARS-CoV-2 from 50.7 % (95 % CI 36.0–62.0 %) in Brazil,2 to 83.5 % (95 % CI 65.4–92.1 %) in Turkey.3 Safety and efficacy data are fundamental for the analysis of a product by regulatory agencies, which decide on authorization for the commercialization and use of the vaccine, but it is data on the effectiveness of the vaccine that allow the evaluation of the product's performance in real life.
The vaccination campaign against COVID-19 in Brazil began in January 2021. As of the second half of the year, studies on the effectiveness of the vaccination program began to be published. The vast majority of them used data routinely collected by national health information systems, specifically the mandatory reporting diseases system and the vaccination registry. Although they are able to provide relevant information, effectiveness studies with secondary data are limited in terms of the completeness and quality of the data, and usually demand probabilistic linking of database records. In order to overcome these limitations, the present study aimed to evaluate the effectiveness of two doses of CoronaVac in preventing symptomatic disease with virological confirmation by SARS-CoV-2, as well as COVID-19 moderate and severe cases.
MethodsA test-negative unmatched case-control design was used, in which cases were patients with suspected COVID-19 with virological confirmation, and controls were those whose test was negative for SARS-CoV-2.
Cases and controls were obtained at primary health care and emergency care clinics or from COVID-19 surveillance database with the onset symptoms registered from March to November 2021 from two municipalities located in the state of São Paulo, Brazil: Campinas (population 1,223,237 in 2021) and Araraquara (population 240,542 in 2021).6
The eligibility criteria were: being a clinically suspected COVID-19 case, according to the Brazilian Ministry of Health definition (presenting at least two of the following symptoms: fever, chills, sore throat, headache, cough, runny nose, olfactory or taste disorders)4; having performed the RT-PCR test with a sample collected within 7 days of the onset of symptoms; being 18 years or older, and having had the chance to receive two doses of the CoronaVac vaccine in the basic scheme, in accordance with the vaccination strategy of the municipality of residence. The exclusion criteria were to have a contraindication to the use of any vaccine against COVID-19; and having received any COVID-19 vaccine other than CoronaVac in the basic scheme.
Definition of cases and controlsCase: Suspected COVID-19 case confirmed by laboratory criteria (positive RT-PCR test result).
Control: Suspected COVID-19 case discarded by laboratory criteria (negative RT-PCR test result).
Clinical severity classification: After their enrollment, participants were classified according to WHO's COVID-19 clinical severity scale.5 Participants with score above 3 were classified as moderate/severe.
Definition of vaccine statusComplete schedule: Having received two doses of CoronaVac in the basic schedule with an interval of at least 14 days between the second dose and the onset of symptoms suggestive of COVID-19.
Unvaccinated: Not having received any dose of COVID-19 vaccine or having received only one dose with an interval of less than 14 days between the first dose and the onset of symptoms suggestive of COVID-19.
Ethical issuesThe study was approved by the Santa Casa de São Paulo ethical review board (statement number: 4.734.648). The participant's informed consent was obtained prior to performing any study procedure. The potential participants or legal guardians were only included in the study after their agreement to participate and signing the Informed Consent Form (ICF). Participants identified in the surveillance databases (retrospective approach) were invited to participate in the study through telephone calls. The calls were recorded, and the ICF was read and explained. The participant's consent was verbally obtained. The study obtained a waiver of the ICF from the IRB to include deceased cases.
ProceduresA screening questionnaire was applied to those individuals who attended the healthcare clinics with a clinical suspicion of COVID-19. After validation of the inclusion and exclusion criteria, eligible participants were invited to participate in the study. Upon acceptance, they proceeded to a face-to-face questionnaire and up to two telephone interviews to update the clinical evolution of the disease, or consultation of their medical records. For participants identified in the databases, a single questionnaire was used, applied by a telephone interview.
Data management and analysisData from the participants’ interviews were recorded in electronic questionnaires, on the REDCap platform (REDCap Consortium, Nashville, TN, USA). Two national health information systems, managed by the Brazilian Ministry of Health and by the State of São Paulo's Health Department, were used to obtain additional data and/or to validate the participants’ responses: the Gerenciamento de Atividades Laboratoriais – GAL (Management of Laboratory Activities, a public health laboratory database), to get the SARS-CoV-2 PCR results, and the VaciVida (State of São Paulo's vaccine registry), to access the COVID-19 vaccine data (vaccine manufacturer and vaccination dates).
Additionally, the participants medical records were consulted, if necessary, to obtain data on the evolution and outcomes of the disease. The data were aggregated into a study database and analyzed using Stata 16.0 (StataCorp LP, College Station, TX, USA), R 4.2 (R Foundation, Vienna, Austria) and SPSS 25.0 (IBM Corp. Released 2017. Armonk, NY, USA).
The measure of effect was the Vaccine Effectiveness (VE). It was calculated by the formula: VE = 1 ‒ Odds Ratio, and presented with its 95 % Confidence Interval. In the effectiveness analysis for symptomatic virologically confirmed COVID-19 cases the dependent variable was the status, as a case or a control. The exposure variable was the vaccination status, and the co-variables were: age, sex, race/ethnicity, educational level, Body Mass Index (BMI), comorbidities, and variables related to exposure to SARS-CoV-2. In the effectiveness for moderate/severe disease, just the cases were included. The dependent variable was a score greater than 3 in the case severity classification, compared to those with a score equal to 3 or lower.5 Participants with incomplete schedule were not included in the VE analysis.
The descriptive analysis was conducted. Differences in proportions between cases and controls were analyzed using the Chi-Square test or χ2 for linear trend. The t-test was used to determine differences between means. Logistic regression analysis was conducted to estimate the crude odds ratio of the dependent variable for each variable of interest. Variables with p < 0.10 or with clinical relevance were incorporated into the regression analysis. Logistic regression analysis for unmatched studies to control for confounding variables was conducted to obtain adjusted odds ratios and their 95 % Confidence Intervals. The variables with p < 0.05, the statistical adjustment variables and those that were deemed of clinical relevance were kept in the final model.
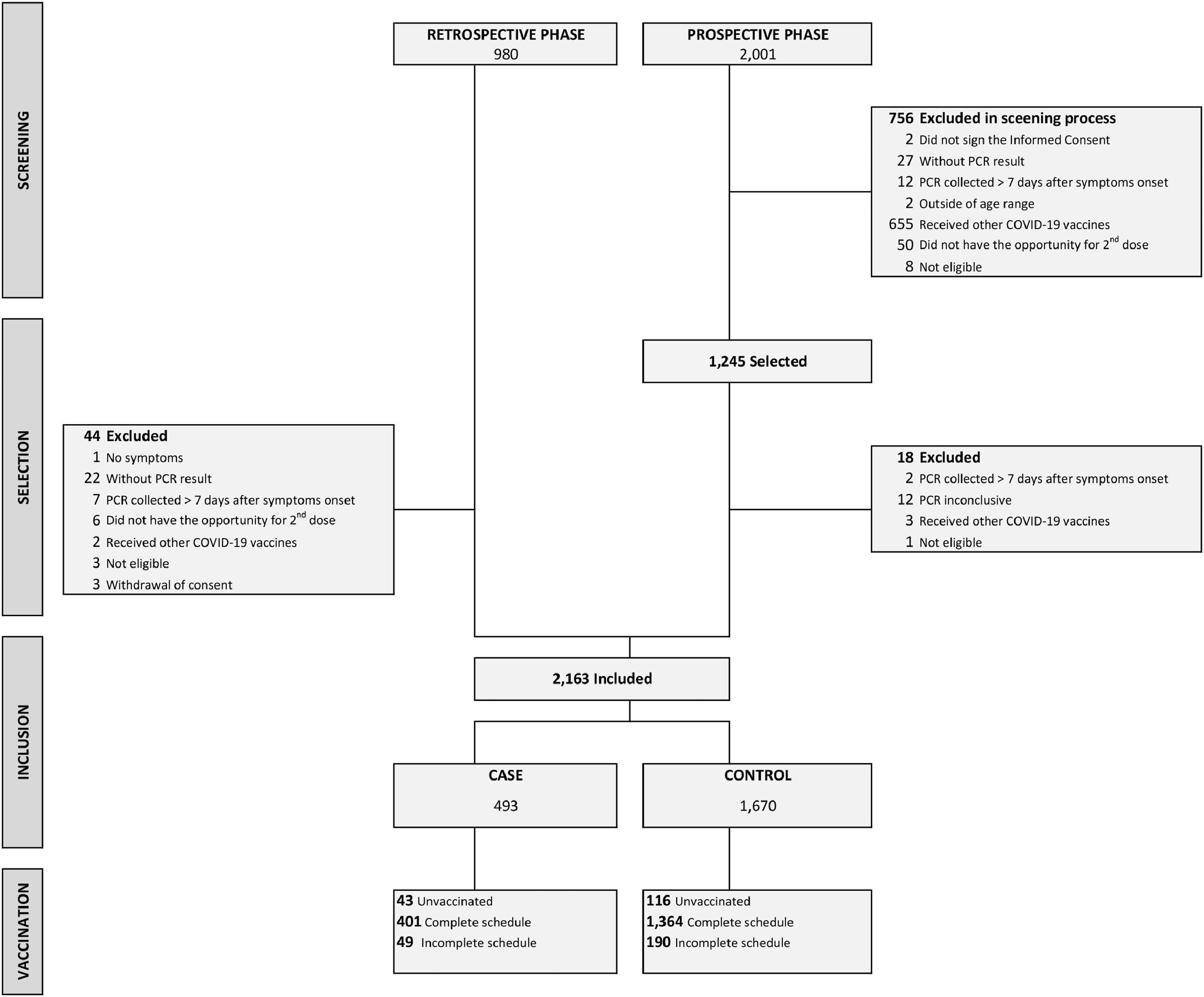
ResultsA total of 2981 potential participants were screened for eligibility, of which 2163 were actually included, being 493 cases and 1670 controls (Fig. 1).
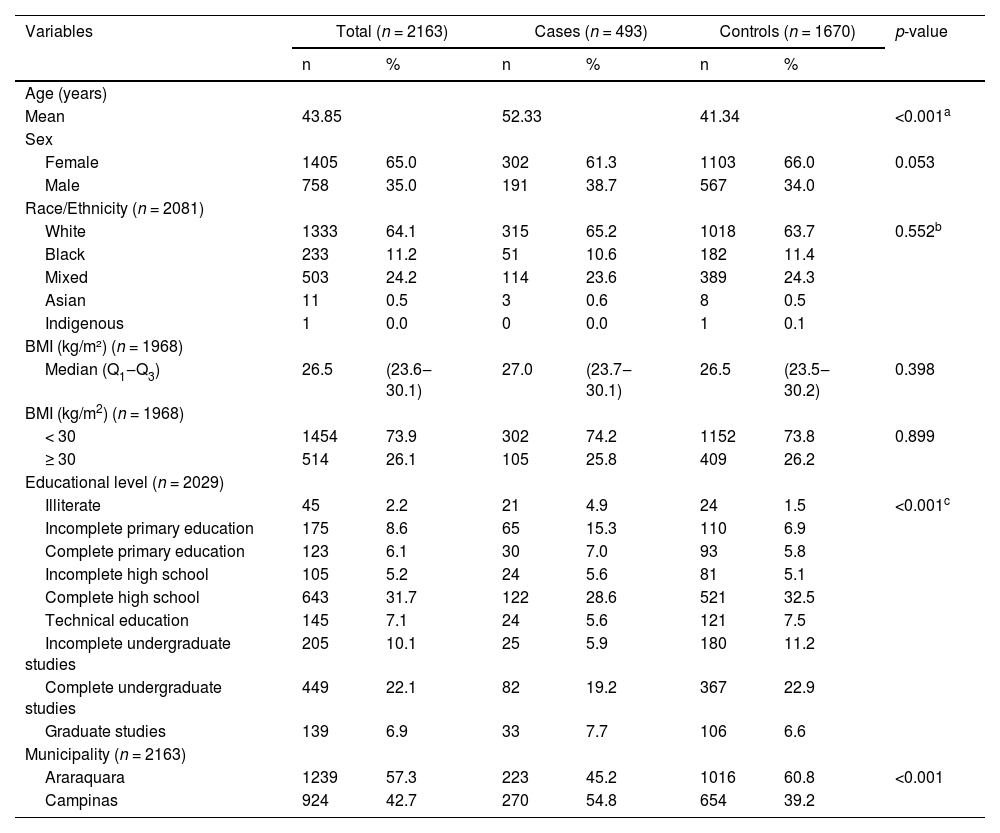
Cases were older and less educated than controls (Table 1). They also were more likely to have had contact with someone with suspected or confirmed COVID-19 in the two weeks before the onset of symptoms (Table 2). Regarding their vaccine status, cases and controls were classified as unvaccinated, vaccinated with a complete schedule, or vaccinated with an incomplete schedule (Table 2). For vaccinated participants, the median interval between the second dose and the onset of symptoms was 110 days (Results are not show).
Characteristics of cases and controls.
| Variables | Total (n = 2163) | Cases (n = 493) | Controls (n = 1670) | p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age (years) | |||||||
| Mean | 43.85 | 52.33 | 41.34 | <0.001a | |||
| Sex | |||||||
| Female | 1405 | 65.0 | 302 | 61.3 | 1103 | 66.0 | 0.053 |
| Male | 758 | 35.0 | 191 | 38.7 | 567 | 34.0 | |
| Race/Ethnicity (n = 2081) | |||||||
| White | 1333 | 64.1 | 315 | 65.2 | 1018 | 63.7 | 0.552b |
| Black | 233 | 11.2 | 51 | 10.6 | 182 | 11.4 | |
| Mixed | 503 | 24.2 | 114 | 23.6 | 389 | 24.3 | |
| Asian | 11 | 0.5 | 3 | 0.6 | 8 | 0.5 | |
| Indigenous | 1 | 0.0 | 0 | 0.0 | 1 | 0.1 | |
| BMI (kg/m²) (n = 1968) | |||||||
| Median (Q1‒Q3) | 26.5 | (23.6‒30.1) | 27.0 | (23.7‒30.1) | 26.5 | (23.5‒30.2) | 0.398 |
| BMI (kg/m2) (n = 1968) | |||||||
| < 30 | 1454 | 73.9 | 302 | 74.2 | 1152 | 73.8 | 0.899 |
| ≥ 30 | 514 | 26.1 | 105 | 25.8 | 409 | 26.2 | |
| Educational level (n = 2029) | |||||||
| Illiterate | 45 | 2.2 | 21 | 4.9 | 24 | 1.5 | <0.001c |
| Incomplete primary education | 175 | 8.6 | 65 | 15.3 | 110 | 6.9 | |
| Complete primary education | 123 | 6.1 | 30 | 7.0 | 93 | 5.8 | |
| Incomplete high school | 105 | 5.2 | 24 | 5.6 | 81 | 5.1 | |
| Complete high school | 643 | 31.7 | 122 | 28.6 | 521 | 32.5 | |
| Technical education | 145 | 7.1 | 24 | 5.6 | 121 | 7.5 | |
| Incomplete undergraduate studies | 205 | 10.1 | 25 | 5.9 | 180 | 11.2 | |
| Complete undergraduate studies | 449 | 22.1 | 82 | 19.2 | 367 | 22.9 | |
| Graduate studies | 139 | 6.9 | 33 | 7.7 | 106 | 6.6 | |
| Municipality (n = 2163) | |||||||
| Araraquara | 1239 | 57.3 | 223 | 45.2 | 1016 | 60.8 | <0.001 |
| Campinas | 924 | 42.7 | 270 | 54.8 | 654 | 39.2 | |
BMI, Body Mass Index, ratio of the individual's weight to the square of his/her height; Q1–Q3, First quartile; Third quartile.
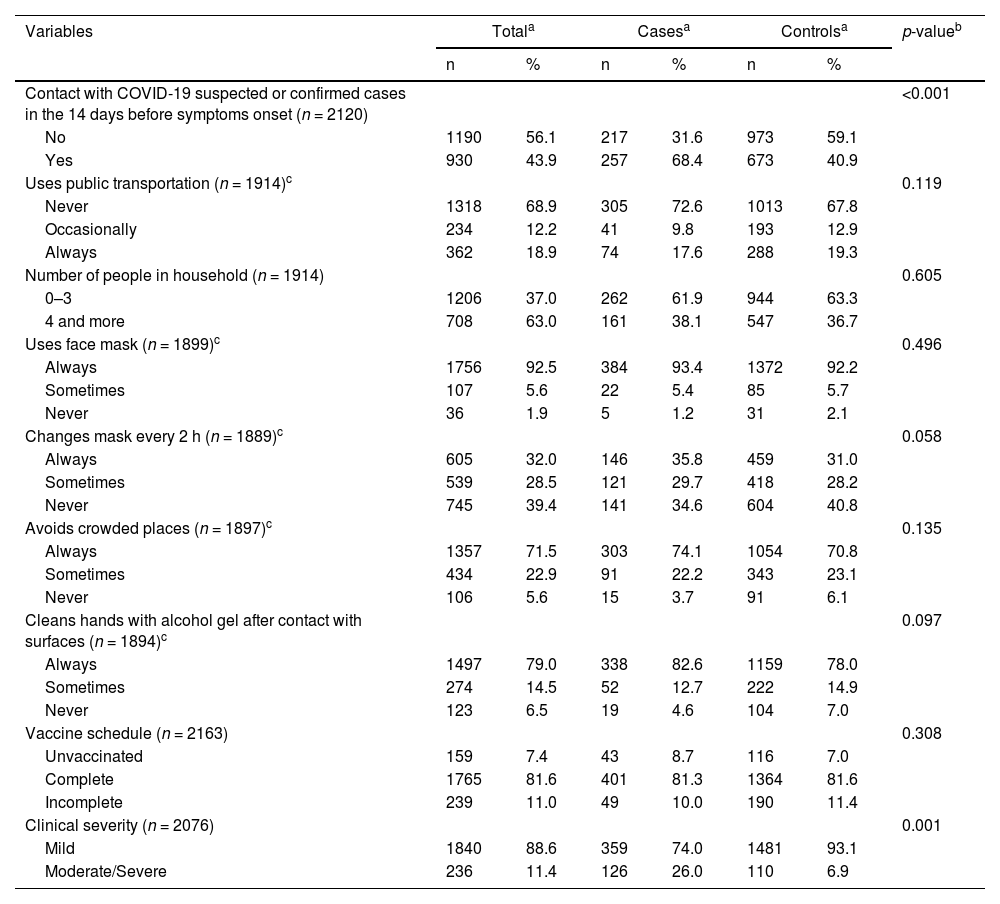
Distribution of cases and controls according to variables related to exposure to SARS-CoV-2 and vaccination with CoronaVac.
| Variables | Totala | Casesa | Controlsa | p-valueb | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Contact with COVID-19 suspected or confirmed cases in the 14 days before symptoms onset (n = 2120) | <0.001 | ||||||
| No | 1190 | 56.1 | 217 | 31.6 | 973 | 59.1 | |
| Yes | 930 | 43.9 | 257 | 68.4 | 673 | 40.9 | |
| Uses public transportation (n = 1914)c | 0.119 | ||||||
| Never | 1318 | 68.9 | 305 | 72.6 | 1013 | 67.8 | |
| Occasionally | 234 | 12.2 | 41 | 9.8 | 193 | 12.9 | |
| Always | 362 | 18.9 | 74 | 17.6 | 288 | 19.3 | |
| Number of people in household (n = 1914) | 0.605 | ||||||
| 0–3 | 1206 | 37.0 | 262 | 61.9 | 944 | 63.3 | |
| 4 and more | 708 | 63.0 | 161 | 38.1 | 547 | 36.7 | |
| Uses face mask (n = 1899)c | 0.496 | ||||||
| Always | 1756 | 92.5 | 384 | 93.4 | 1372 | 92.2 | |
| Sometimes | 107 | 5.6 | 22 | 5.4 | 85 | 5.7 | |
| Never | 36 | 1.9 | 5 | 1.2 | 31 | 2.1 | |
| Changes mask every 2 h (n = 1889)c | 0.058 | ||||||
| Always | 605 | 32.0 | 146 | 35.8 | 459 | 31.0 | |
| Sometimes | 539 | 28.5 | 121 | 29.7 | 418 | 28.2 | |
| Never | 745 | 39.4 | 141 | 34.6 | 604 | 40.8 | |
| Avoids crowded places (n = 1897)c | 0.135 | ||||||
| Always | 1357 | 71.5 | 303 | 74.1 | 1054 | 70.8 | |
| Sometimes | 434 | 22.9 | 91 | 22.2 | 343 | 23.1 | |
| Never | 106 | 5.6 | 15 | 3.7 | 91 | 6.1 | |
| Cleans hands with alcohol gel after contact with surfaces (n = 1894)c | 0.097 | ||||||
| Always | 1497 | 79.0 | 338 | 82.6 | 1159 | 78.0 | |
| Sometimes | 274 | 14.5 | 52 | 12.7 | 222 | 14.9 | |
| Never | 123 | 6.5 | 19 | 4.6 | 104 | 7.0 | |
| Vaccine schedule (n = 2163) | 0.308 | ||||||
| Unvaccinated | 159 | 7.4 | 43 | 8.7 | 116 | 7.0 | |
| Complete | 1765 | 81.6 | 401 | 81.3 | 1364 | 81.6 | |
| Incomplete | 239 | 11.0 | 49 | 10.0 | 190 | 11.4 | |
| Clinical severity (n = 2076) | 0.001 | ||||||
| Mild | 1840 | 88.6 | 359 | 74.0 | 1481 | 93.1 | |
| Moderate/Severe | 236 | 11.4 | 126 | 26.0 | 110 | 6.9 | |
Cases were more likely to refer chronic comorbidities than controls: diabetes mellitus, hypertension, heart disease and pulmonary disease (Table S1).
In the univariate analysis, the variables that were significantly associated with the odds of being a case were age, the educational level, any previous comorbidity, and the reported contact with a COVID-19 suspected or confirmed case in the 14 days before symptoms onset (Tables 1, 2 and S1). Regarding the clinical severity classification, the vast majority of controls were classified as mild. As for COVID-19 cases, almost a quarter of them were classified as moderate/severe (Table 2 and S2).
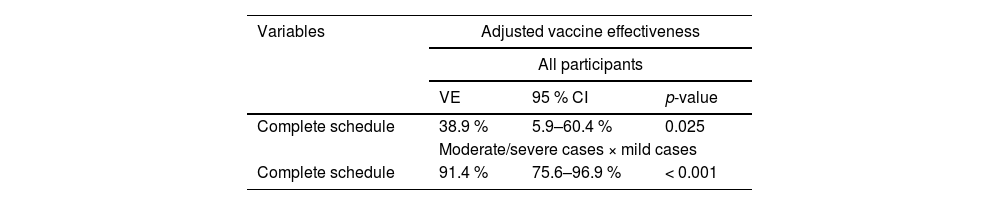
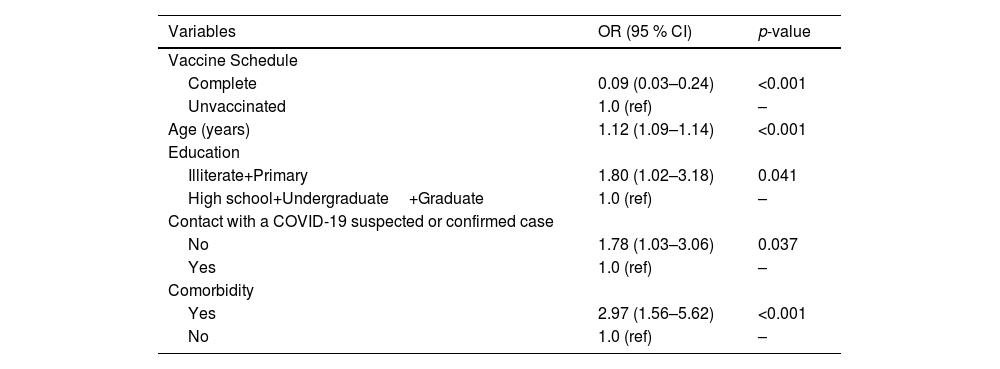
In the multivariate logistic regression analysis, the age, the reported contact with a COVID-19 suspected or confirmed case in the 14 days before symptoms onset, and the educational level remained in the model, being independently associated with the outcome (Table 3). Finally, the Adjusted Vaccine Effectiveness (AVE) for symptomatic virologically confirmed COVID-19 was 38.9 % (95 % CI 5.9 %‒60.4 %) (Table 4).
Adjusted odds ratio for the association of independent variables and cases/controls.
The multivariate model was adjusted comparing moderate/severe cases to the mild ones. In this analysis, vaccination with complete schedule was independently associated with severity, along with age, educational level, contact with a suspected or confirmed case in the 14 days before symptoms onset, and reporting any comorbidity (Table 5). Adjusted vaccine effectiveness for moderate/severe cases was 91.4 % (95 % CI 75.6 %‒96.9 %) with a complete schedule (Table 4).
Adjusted odds ratio for the association of independent variables and moderate/severe cases and mild cases × controls.
In this test-negative case-control study, effectiveness of CoronaVac in the prevention of symptomatic virologically confirmed COVID-19 was 39 %. These figures are in line with the results of other CoronaVac effectiveness studies. In the State of São Paulo, Brazil, in early 2021, effectiveness of CoronaVac among older adults was estimated in 46.8 % (95 % CI 38.7–53.8 %).7 In Hong Kong, the effectiveness of CoronaVac in the prevention of mild to moderate disease among adults from 20 to 59 years of age was 25.1 % (95 % CI 14.7–34.3 %).8 In Guangdong, China, effectiveness against infection was 60.4 % (95 % CI 31.8–88.9 %) during an outbreak of the Delta variant.9 However, in Chile, the effectiveness of CoronaVac in the prevention of symptomatic virologically confirmed COVID-19 was higher (VE = 65.9 %; 95 % CI 65.2–66.6 %) in a study conducted in a short term after the vaccination campaign.10
Regarding moderate/severe disease, a high effectiveness was observed. Our result is consistent with the results of other studies. In a large study carried out with secondary data in Brazil, the effectiveness of CoronaVac for hospitalization due to COVID-19 was 95.6 % (95 % CI 82.4–99.9 %).11 Among older adults in the State of São Paulo, Brazil, effectiveness was 77.6 % against hospitalization, and 83.9 % against death.7 In Chile, effectiveness was 87.5 %, 90.3 % and 86.3 % against hospitalization, ICU admission, and death, respectively [10] while in Hong Kong, effectiveness of Coronavac in the prevention of severe disease and death was 69.9 %.8
Effectiveness estimates are usually lower than efficacy figures obtained in clinical trials. Efficacy of CoronaVac was 50.7 % in a study among frontline healthcare workers in Brazil (Palácios et al.); 65.3 % and 83.5 % among healthy adults from 18 to 59 years of age in Indonesia and Turkey, respectively.3,12 It has been documented that SARS-CoV-2 vaccines protection wanes over time.13 A study carried out in China showed that the effectiveness of CoronaVac against symptomatic infection decreased after six months, but remained high for serious outcomes, such as death.14
When interpreting and comparing different effectiveness studies, it is important to consider some aspects that may influence their results. The design of the study, the analyzed outcomes, the population or population subgroup included the period when the study was carried out, in relation to the evolution of the epidemic, as well as the follow-up time of the participants, should be taken into account. The present study was conducted right after the rollout of SARS-CoV-2 vaccines in Brazil, in early 2021. During the first trimester of 2021 Brazil experienced a large increase in the number of new COVID-19 cases and deaths, corresponding to the emergence of the Gamma variant.15 With time and the increase in vaccination coverage, the Gamma variant waned, and there was a drop in the number of cases in the second half of the year, when the field work of our prospective phase begun, and the predominant variant shifted to Delta. Older adults were the among the priority groups for vaccination, being the first ones to be vaccinated. This may have reduced the incidence and severity of cases among the population over 60 years of age, which accounted for just 20 % of study participants (data not shown). Regarding the follow-up time, among the participants for whom this variable can be computed, it was between 15 and 16 weeks. The study design in the prospective phase allowed the inclusion of a large proportion of eligible patients, the number of refusals was minimal.
In addition to the limitations arising from the observational design, our study has some others that deserve to be mentioned. Our sample size was small, not allowing us to estimate effectiveness by age group, especially for the older population. The number of severe cases was also small, and there was a higher concentration of deaths among subjects identified from the surveillance database, as the IRB exempted us from obtaining the informed consent form for their inclusion in the study.
In summary, the study demonstrated the high effectiveness of CoronaVac in preventing moderate and severe cases of COVID-19. Its effectiveness against virologically confirmed symptomatic infection was within expectations, similar to that observed in other studies, in the pre-Omicron period. The inactivated vaccine platform is very well known, representing lower costs than other COVID-19 vaccine's platforms, and has a high potential and capability for the national manufacturing to provide sustainable supply to the Ministry of Health.
The authors are thankful to the study participants, to the field teams, and to Dr. Alexander Precioso, Dr. Juliana Viscondi, and Dr. Anderson da Silva for their contributions for the study implementation.