To analyse the prevalent microorganisms and their antimicrobial resistance among intensive care unit patients in a tertiary care centre in New Delhi.
MethodsA retrospective study of all consecutive blood cultures from various intensive care unit patients in the hospital during four years (January 2008 to December 2011). Antibiotic consumption data in the intensive care units were also analysed during the same period.
ResultsOut of the total 22,491 blood cultures processed, 2846 samples were positive and 3771 microorganisms were isolated. The blood culture positivity was estimated as 12.7% of which 67.5% were monomicrobial and 32.5% polymicrobial infections. Gram negative bacilli, Gram positive cocci, and fungi were isolated in 49%, 33%, and 18% cases, respectively. Coagulase negative staphylococcus was the commonest single isolate followed by Candida spp. A drastic shift in the distribution of Candida spp. towards nonalbicans along with high resistance to azole group of antifungals suggest echinocandins for the empiric therapy of candidemia. High penicillin resistance in Gram positive isolates suggest vancomycin, linezolid and tigecycline as the options for empiric therapy, whereas tigecycline and colistin are the only options remaining for highly resistant Gram negative isolates. Aminoglycosides were observed to have better sensitivity and reduced usage when compared with cephalosporins and β-lactam+β-lactam inhibitor combinations.
ConclusionsHigh frequencies of multidrug resistant organisms were observed in intensive care units which is a warning as to use the only few effective antimicrobials wisely to reduce selective pressure on sensitive strains.
Infections caused by multidrug resistant bacteria constitute a serious problem for intensive care patients throughout the world. Prevalent pathogens and their antimicrobial resistance pattern may vary in different intensive care units (ICUs) depending upon the antibiotic pressure in that health care facility. This information could be beneficial in choosing the appropriate antimicrobials, which in turn can reduce the length of stay as well as the morbidity and mortality in ICU patients.1
The paradox remains where the antibiotics are needed the most (ICU), there the highest resistance, is observed due to multiple reasons. Random use of high-end antimicrobials has the risk of eventually selecting out mutants that are multidrug resistant. Every hospital must recognize the epidemiology of its microorganisms to recommend initial appropriate presumptive antimicrobial therapy. The application of hospital-wide antibiograms to guide clinicians in the initial choice of antimicrobials is the usual approach adopted. If more resistant organisms are isolated from ICU patients, this important information would be masked by the use of hospital-wide antibiogram.
Here we have made an attempt to analyse the prevalent microorganisms causing blood stream infection (BSI) and their antimicrobial resistance as seen among the ICU patients in a tertiary care centre in New Delhi.
Materials and methodsPatientsThis retrospective study was conducted for a period of four years (January 2008 to December 2011) in a 650-bed tertiary care centre in New Delhi. A total of 22,491 blood samples were analysed from the patients admitted in various ICUs in the hospital, which include general adult ICU, coronary care unit (CCU), surgical ICU, and the liver, kidney and bone marrow transplant ICUs.
Sample collection and processingFive to 10mL of blood sample was collected and were inoculated immediately into BacT/ALERT culture bottles (bioMerieux, Durham, North Carolina, USA) under complete aseptic conditions and were processed as per the manufacturer's specifications.
Identification and susceptibility testing of isolatesPositive blood culture isolates were identified using VITEK 2 automated system (bioMerieux, Durham, North Carolina). MIC was confirmed using E-test (bioMerieux, France) in case of VRE. In case of any discrepancy E-test result was taken as final. Susceptibility testing was performed by Kirby Bauer method for tigecycline (15μg, Oxoid Ltd., Basingstoke, Hampshire, England), and colistin (10μg, HiMedia Laboratories, Mumbai) and results interpreted as per Clinical Laboratory Standards Institute (CLSI) guidelines2 and British society for Antimicrobial Chemotherapy (BSAC) guidelines where ever CLSI guidelines were not available.3 Daptomycin MIC was determined for Staphylococcus aureus and Enterococcus spp. using E-test during the period of 2010 and 2011. Antibiotic resistance data were extracted from the hospital information system (Intersystems, Cambridge, MA, USA) using a software Speedminer (Petaling Jaya, Malaysia) and analysed using a customized software, Wattal-Protech, Delhi, India.
Screening for carbapenem resistanceBecause of the observation of high carbapenem resistance among Enterobacteriaceae at our facility, we tried to assess the magnitude of different mechanisms of carbapenem resistance. All consecutive isolates of Enterobacteriaceae were screened for carbapenem resistance by modified Hodge test (MHT) during the period of December 2010 to April 2011. All MHT positive isolates were further tested for metallo-β-lactamase (MBL) by MBL E-test strips (bioMerieux, France). Further confirmation of all MBL positive isolates for New Delhi Metallo-beta-lactamases (NDM-1) was done using a real time polymerase chain reaction (PCR) assay for the detection of the blaNDM-1 using Taq Man probes.4
Antifungal susceptibility testingAntifungal susceptibility testing was performed against amphotericin B, 5-flucytosine, fluconazole, itraconazole, and voriconazole by broth microdilution using API system (ATB FUNGUS 3, bioMerieux, France), during 2008. For isolates wherein API system was not recommended by the manufacturer, sensitivity was done using E-test for amphotericin B, fluconazole, and voriconazole. During 2009–2011, antifungal susceptibility testing was done using VITEK 2 automated system except for caspofungin, which was done using E-test. Results were interpreted as per the CLSI guidelines.5
Antimicrobial consumption dataFour years antimicrobial consumption data were evaluated from the antibiotic dispensing data from the hospital information system. Customized software Speedminer was used to extract the data from the hospital information system. The amount of antimicrobial drug in grams was converted into the number of defined daily doses (DDDs)/100 bed-days using ABC calc.6
Ethical considerationsThe study was approved by the hospital ethics committee (reference number EC/09/12/414 and approval letter dated 31/01/2013). Consent was waived since this was an anonymised study with retrospective evaluation.
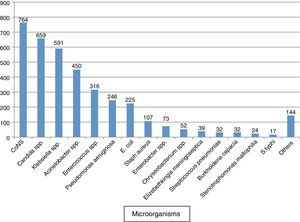
ResultsA total of 2846 samples were positive from 22,491 blood cultures received from the study group. The blood culture positivity rate in our ICUs was estimated to be 12.7%. A total of 3771 microorganisms were isolated from 2846 episodes of BSIs. 67.5% (1921/2846) of infections were monomicrobial, while 32.5% (925/2846) were polymicrobial (Fig. 1).
Analysis of these isolates showed that the commonest single isolate among our ICU patients was coagulase negative staphylococcus (CoNS) (20.3%) followed by Candida spp. (17.5%). Gram negative bacilli (GNB), Gram positive cocci (GPC), and fungi were isolated in 49%, 33%, and 18% cases, respectively. Among GNB, Klebsiella spp. was the commonest followed by Acinetobacter spp. Candida tropicalis and Candida haemulonii were the most frequently isolated Candida spp.
Antimicrobial susceptibility testingPatterns of antimicrobial susceptibility for the major pathogens are shown in Tables 1 and 2. The evidence generated here indicates that antibiotics like penicillins and most cephalosporins would not be effective against Gram positive and negative bacteria in the ICU. For highly resistant bacteria, especially Klebsiella spp. and Acinetobacter spp., which were also the commonest gram negative isolates in our ICUs even β-lactam+β-lactam inhibitor (BL+BLI) combinations and carbapenems were not effective. Amikacin was slightly better and the only remaining effective antibiotics were tigecycline and colistin.
Percentage resistance of antimicrobial agents in the Gram negative bacilli.
| Percentage resistance | ||||
|---|---|---|---|---|
| E. coli | Klebsiella spp. | Acinetobacter spp. | Pseudomonas aeruginosa | |
| Ampicillin | 96 | NT | 99 | NT |
| Ceftriaxone | 91 | 96 | 99 | NT |
| Ceftazidime | NT | NT | NT | 58 |
| Cefepime | 90 | 95 | 94 | 60 |
| Gentamicin | 64 | 88 | 86 | 73 |
| Amikacin | 20 | 66 | 78 | 68 |
| Ciprofloxacin | 93 | 91 | 89 | 71 |
| Piperacillin+tazobactam | 49 | 85 | 90 | 45 |
| Cefoperazone+sulbactum | 35 | 82 | 79 | 64 |
| Ertapenem | 13 | 70 | NT | NT |
| Imipenem/meropenem | 12 | 77 | 83 | 67 |
| Tigecycline | 0 | 28 | 47 | NT |
| Colistin | 0 | 0 | 0.5 | 0 |
ICU, Intensive care unit; NT, not tested.
Percentage resistance of Gram positive bacteria to antimicrobial agents.
| Antimicrobial agent | Percentage resistance | |||
|---|---|---|---|---|
| S. aureus | CoNS | Enterococcus spp. | Strep. pneumoniae | |
| Penicillin | 100 | 99.5 | NT | 16 |
| Ampicillin | NT | NT | 77 | NT |
| Oxacillin | 47 | 94 | NT | NT |
| Ceftriaxone | NT | NT | NT | 0 |
| Clindamycin | 49 | 80 | NT | NT |
| Levofloxacin | NT | NT | NT | 0 |
| Gentamicin | 56 | 72 | NT | NT |
| Gentamicin120 | NT | NT | 84 | NT |
| Vancomycin | 0 | 0 | 23 | 0 |
| Linezolid | 0 | NT | 0 | NT |
| Tigecycline | 0 | NT | 0 | NT |
| Daptomycin | 0 | NT | 0 | NT |
ICU, intensive care unit; CoNS, coagulase negative staphylococcus; NT, not tested.
A total of 34 Enterobacteriaceae isolates (7 Escherichia coli and 27 Klebsiella pneumoniae) were subjected for carbapenemase screening. The overall prevalence of carbapenemase production (MHT positive isolates), MBLs (MBL E-test positive isolates) and KPC (MHT positive and MBL negative) was 52.9%, 41.2% and 11.8%, respectively (Table 5). Interestingly all the phenotypically MBL positive isolates, tested for NDM-1 by PCR were positive, implying 100% concordance between the phenotypic presence of MBLs and presence of blaNDM-1. Fourteen K. pneumoniae (51.9%) and no E. coli isolates were observed to be NDM-1 producers.
The amount of antimicrobials used during the same time period is given in Table 3. The distribution of various candida species and their antifungal susceptibility pattern are given in Table 4.
Antimicrobial use during 2008–2011.
| Antimicrobial class | DDD/100 bed days |
|---|---|
| Penicillins | 3.62 |
| Total cephalosporins | 25.92 |
| Total BL–BLI combinations | 27.62 |
| Quinolones | 10.45 |
| Aminoglycosides | 8.60 |
| Clindamycin | 8.13 |
| Carbapenem | 17.38 |
| Glycopeptides | 10.11 |
| Linezolid | 1.72 |
| Colistin | 6.23 |
| Tigecycline | 2.74 |
| Total | 122.52 |
DDD, defined daily doses; BL+BLI=β-lactam+β-lactam inhibit.
Percentage resistance of common candida isolates to antifungals.
| Most common pathogens (n=) | Antifungal (percentage resistance) |
|---|---|
| C. tropicalis (130) | Amphotericin B (0), Caspofungin (0), Voriconazole (1.2), Fluconazole (3.6), Flucytosine (6.5) |
| C. haemulonii (118) | Flucytosine (21.3), Caspofungin (4.9), Voriconazole (20.7), Fluconazole (96.7), Amphotericin B (98.1) |
| C. parapsilosis (109) | Amphotericin B (0), Flucytosine (1.4), Caspofungin (0), Voriconazole (17), Fluconazole (37.4) |
| C. albicans (104) | Amphotericin B (0), Caspofungin (0), Voriconazole (0), Fluconazole (0.8), Flucytosine (2.5) |
| C. glabrata (55) | Amphotericin B (0), Flucytosine (0), Caspofungin (0), Voriconazole (13.6), Fluconazole (25.8) |
Mechanism of carbapenem resistance among Enterobacteriaceae (December 2010 to April 2011).
| MHT positive | MHT positive and MBL positive (NDM-1) | MHT positive and MBL negative (KPC) | |
|---|---|---|---|
| E. coli | 1 (4.3) | 0 (0) | 1 (14.3) |
| K. pneumoniae | 17 (63) | 14 (51.9) | 3 (11.1) |
MHT, modified Hodge test; MBL, metallo-β-lactamase. Figures in parenthesis denotes percentage.
Keeping in view the source of patients to our ICU being from all over the city and other parts of the country including tertiary care centres, this ecology could be representative of most of the ICUs in the country. In our study, coagulase negative staphylococci were the most common blood culture isolate (20.3%) (Fig. 1). Some other studies in our country (Mukherjee et al., 61%) as well as from other countries (Japoni et al., 67.7%, Karlowsky et al., 42%) also observed CoNS as the commonest blood stream isolate.7–9 In a review by Valles et al. who reviewed more than five recent publications from different parts of the world also observed CoNS as the commonest microorganism (20–30%) causing BSIs among ICU patients.1 Appropriate antimicrobial therapy and control measures could be adopted to prevent cross infection of multidrug-resistant CoNS bacteria from patient-to-patient and ICU environment. Moreover, CoNS isolates are often skin colonizers and appear in blood culture as common contaminants at the time of sample collection.10 Meticulous skin disinfection at the time of venepuncture can limit this potential contaminant species to a large extent. Clinical correlation of the blood culture isolate and determination of time to positivity of CoNS isolates can help to differentiate between potential contaminants and true pathogens.11,12
Considering the fact that CoNS isolated from blood cultures are often contaminants,10Candida spp. (17.5%) was the commonest single isolate in the current study (Fig. 1). Studies have proven the various risk factors associated with candida infection in ICU patients which include exposure to multiple antibiotics, indwelling catheters, parenteral nutrition, previous surgery and presence of a solid organ malignancy.13 Candida remains a major cause of BSIs in ICU patients worldwide.14,15 In fact a major increase in candida BSI started during the 1980s.16 According to the surveillance data from the US Centers for Disease Control and Prevention (CDC), candida accounts for 12% of all hospital-acquired BSIs.17 Another interesting observation is the shift in the distribution of Candida spp. responsible for BSI to non-albicans species. In our study Candida albicans accounted for only 15.3% of candida BSIs. This epidemiologic trend has also been observed in many studies in different parts of the world.18,19 This could be attributed to the increased use of fluconazole as observed in another study in our hospital.20 Even though C. tropicalis, the commonest candida isolate in our ICUs still remains susceptible to most of the antifungals, C. haemulonii, the 2nd commonest isolate, has shown very high resistance to fluconazole and even to amphotericin B. In other studies also, C. haemulonii has shown increased MICs and frank resistance to both amphotericin B and fluconazole and has resulted in clinical failure.21 Our susceptibility data suggests that reduced susceptibility to fluconazole is common in Candida glabrata and Candida parapsilosis, and it may prove simpler in clinical practice to use an alternative agent to treat candidaemia due to these species. Reduced susceptibility to fluconazole in C. glabrata was consistent with previously reported data.22,23 In contrast, C. parapsilosis has usually been reported to be sensitive to azoles.
Enterococcus spp. also was observed as an important pathogen in our study (8.4%). Enterococci form part of the normal flora of the gastrointestinal tract and female genitourinary tract. Over the past decade, vancomycin resistant enterococci (VRE) have emerged as a leading cause of nosocomial infections due to its spread by direct patient-to-patient contact and by indirect transmission via hospital personnel, environmental surfaces and hospital equipment. In our study, 23% of enterococci were VRE, while Japoni et al. reported 10.5% VRE among blood stream isolates in ICU.8 A study by the CDC also reported high prevalence of VRE (29%), similar to our study, among the enterococcal infections in ICU settings.24 Still vancomycin can serve as an important antibiotic in our ICU to control CoNS, S. aureus and streptococcal infections. In the case of S. aureus and CoNS, 47% and 94% of isolates were resistant to methicillin respectively. Our findings are similar to other studies that report an MRSA rate in ICU varying between 47 and 65%.9,25,26 All the MRSA and VRE isolates in our study were sensitive to vancomycin/teicoplanin, linezolid, tigecycline as well as daptomycin.
Penicillin resistance was observed in 16% (five isolates) of S. pneumoniae isolates, all of which were having an MIC in the moderately sensitive range (MIC 0.25–1μg/mL). The rate of resistance was similar to some other studies in India quoting 5–18% penicillin resistance.27–29 There was no frank penicillin resistance or multidrug resistance, unlike some other studies from India.28,29 All the isolates were sensitive to ceftriaxone and quinolones.
Gram negative bacteria caused more than half of the BSIs in the current study of ICUs. This observation in our study is in agreement with many other studies from India as well as from different parts of the world, even though the distribution of various Gram negative pathogens tend to vary among the different ICUs.30,31 This study documents the extent of drug resistance among the frequently isolated Gram negative isolates from intensive care patients. An interesting observation of this analysis is the comparatively better sensitivity of aminoglycoside against the majority of GNB. Similar observations of better aminoglycoside sensitivity (50–60%) among respiratory isolates was observed by other authors as well.32,33 High resistance to cephalosporins and BL+BLI combinations observed in our study might be due to the selective influence of extensive usage of these antimicrobials as compared to aminoglycoside (see Table 3). Our results show a high prevalence of carbapenem resistance among non-fermenters, higher in Acinetobacter spp. (83%) followed by Pseudomonas aeruginosa (67%). Carbapenem resistance observed in Acinetobacter isolates was much higher compared to some recent studies in ICUs where the resistance is reported to be 16–17%.8,30,34 The trend of carbapenem resistance in the past decade indicates significant increase in resistance in Acinetobacter spp. with no significant change in P. aeruginosa.35,36 Our study shows that, among Enterobacteriaceae, K. pneumoniae has emerged as a problematic species in our health care settings as compared to E. coli due to high prevalence of carbapenemases, which are predominantly NDM-1. Though KPC was previously thought to be the main mechanism of resistance among Enterobacteriaceae, the data in our study shows that MBL has replaced other mechanism of carbapenem resistance. In-fact all isolates positive for MBL phenotypically were also positive for NDM-1 by PCR. The rapidly emerging NDM-1 is of concern from the perspective of infection control not only because of their broad β-lactam resistance coverage but also due to their transmissibility. Additionally we tested few strains of non-fermenters (20 P. aeruginosa and seven Acinetobacter spp.) for the presence of blaNDM-1. Surprisingly we detected the presence of blaNDM-1 in one isolate of Acinetobacter baumannii. Sporadic cases of NDM-1 producing acinetobacter strains have also been reported around the world37 highlighting its spread to non-fermenters also. Tigecycline, introduced in 2006 in our hospital for MDR bacteria, has lost its effectiveness in 47% of Acinetobacter spp. and 28% of Klebsiella spp. among our blood stream isolates in ICU. Currently, colistin remains the only therapeutic option for the majority of ICU patients, although high-quality pharmacokinetic data and clinical outcome studies are lacking for polymyxin therapy. Among Enterobacteriaceae, Klebsiella spp. with 77% carbapenem resistance appears to be at par with the Acinetobacter spp. (83%). The rapidly increasing prevalence of Enterobacteriaceae harbouring carbapenemases is alarming. Some other recent studies have also reported high carbapenem resistance (77.8%) in K. pneumonia from ICU.38
On studying the pattern of consumption of various antibiotics in the ICUs, an increase in the usage of second-line antibiotics with broad spectrum of activity and reserved for multidrug resistant organisms was observed in our ICUs. The selection pressure associated with the use of high-end antimicrobials might have led to the predominance of multidrug resistant isolates in our ICUs. Even though a direct relationship between the quantity of antimicrobials used and the development of resistance is not easy to establish, studies have documented that the indiscriminate use of antimicrobials has led to the selection of drug-resistant organisms.35,39 An increase in the prevalence of non-fermenters might have led to an increase in the usage of second-line antibiotics such as β-lactam/inhibitor combinations and carbapenems.
In conclusion, BSI caused by multidrug resistant pathogens including, MRSA, VRE, carbapenem resistant Enterobacteriaceae, non-fermenters and non-albicans Candida spp. comprise the current infection profile of the ICUs. Linezolid, tigecycline and vancomycin are the most reliable treatment options for GPC and colistin alone or in combination with carbapenems or aminoglycosides appear to be the remaining treatment options for such Gram negative infections. De-escalation of the high-end antimicrobials once the sensitivity pattern of the isolate is known is suggested to reduce the antimicrobial pressure. More aggressive measures such as routine screening cultures to identify and isolate carriers and the various environmental sources are also recommended, as well as periodical antibacterial sensitivity assessment in ICUs because of the continuous changes in the antibacterial susceptibility patterns.
Conflicts of interestThe authors declare no conflicts of interest.
The study was partially supported by the research committee of the hospital. Authors acknowledge the financial support of Sir Ganga Ram Hospital.