Polymyxin B is one of the last resort option for carbapenem-resistant Klebsiella pneumoniae (CRKP) bloodstream infection in China. Therefore, the timing of administration of polymyxin is frequently delayed. We collected 40 cases of CRKP bloodstream infections (BSIs) treated with combinations based on polymyxin B over 30 months. The primary outcome, 30-day mortality rate, was 52.5% (21/40). Early administration of polymyxin B is meant to administer the drug within 48h of diagnosing bacteremia. Delayed administration was considered when polymyxin B was administered after 48h of bacteremia onset. Polymyxin B duration and total dosages were similar in the two groups (11.57 days versus 11.76 days, p=0.919; 1306.52mg versus 1247.06mg, p=0.711). Compared with delayed administration, early use of polymyxin B-based combination therapy had a significant increase in the rate of bacterial clearance (65.22% versus 29.41%, p=0.025; OR=0.533) and decreased 30-day mortality (39.13% versus 70.59%, p=0.045; OR=0.461) and overall mortality (43.48% versus 82.35%, p=0.022; OR=0.321).
The increasing prevalence of carbapenem-resistant Klebsiella pneumoniae (CRKP) is a healthcare crisis in China. Bloodstream infection (BSI) caused by CRKP is a more serious situation due to ineffective antibacterials and high mortality.1 Therapeutic options against these infections are limited; “second line” agents include colistin, tigecycline, aminoglycosides, and carbapenems.2 The best available treatment against CRKP infections is still under study owing to different chemoresistance mechanisms and limited effectiveness. Rather than monotherapy, clinicians are increasingly resorting to combination therapies, such as dual carbapenems, carbapenems combined with polymyxin B or tigecycline.3–5 However, clinical outcomes are not confirmed in the absence of evidence-based trials.
Regarded as one of the last resorts, polymyxin B has been used alternatively in China, especially following the surge of CRKP only susceptible to colistin. Thus, researchers are paying more attention to polymyxin B options as monotherapy or combination therapy, medication doses, and adverse effects.2 Combination therapy is probably better than monotherapy for serious CRKP bacteremia, but the timing clinicians should implement such therapy is still unclear.2,5,6 Our retrospective observational study aimed to estimate the impact of early initiation of polymyxin B on the outcome of patients with CRKP BSI.
MethodsSettingA 1200-bed academic tertiary care hospital with 40 intensive care beds, in Hangzhou, China. Records from patients with positive blood culture for CRKP were retrieved from October 1st 2015 to March 31st 2018. Patients who received polymyxin B treatment were selected from the hospital database and information on basic characteristics, laboratory results, therapeutic options, and outcomes was abstracted. The clinical parameters, originating from the hospital database without subjective judgments, were nearly objective, which avoided recall bias and selection bias. The key outcome was 30-day mortality, and secondary outcomes included bacterial clearance rate and total mortality. The writing process for this report followed STROBE recommendations.
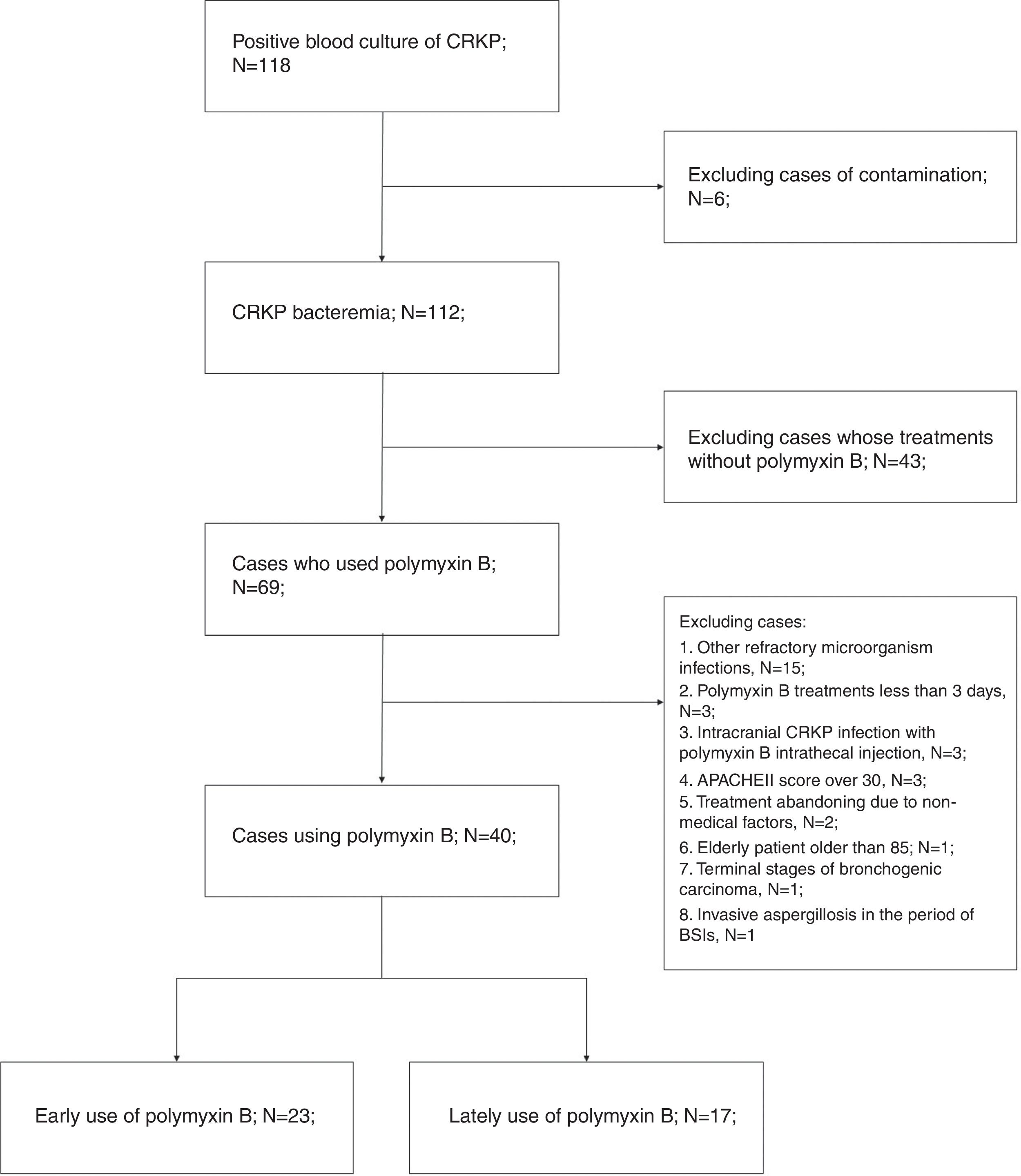
Exclusion criteria included the following: (a) age less than 18 or older than 85 years; (b) pregnancy or breast-feeding women; (c) end-stage renal disease; terminal stages of solid tumor or hematological malignancies; terminal pulmonary diseases; or severe autoimmune disease; (d) other refractory microbial infections in the period of BSIs, including carbapenem-resistant Acinetobacter baumannii (CRAB) and Pseudomonas aeruginosa (CRPA) and other Carbapenem-resistant Enterobacteriaceae (CRE) infections; methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) infections; or invasive fungal disease; (e) polymyxin B treatments for less than three days for any reason; (f) intracranial CRKP infections; and (g) Acute Physiology, Age and Chronic Health Evaluation (APACHE II) score over 30.
DefinitionEarly administration of polymyxin B was considered when the drug was administered within 48h of bacteremia onset; the remaining patients were grouped as delayed administration.5 Bacterial clearance was defined as two negative blood cultures, improved infection symptoms after treatment, and no rebound in one week thereafter All patients received polymyxin B at dosages of 1.5–2.5mg/kg daily. Adult patients received half of the dosage every 12h, with adjusted dosages in patients with renal insufficiency. The diagnostic criteria for acute renal injury (AKI) followed the 2012 Kidney Disease Improving Global Outcomes guidelines. Invasive procedures included catheterizations of deep veins or arteries for continuous renal replacement therapy (CRRT), pulse indicator continuous cardiac output (PICCO), and extracorporeal membrane oxygenation (ECMO). Immunodeficiency was determined mainly by laboratory indicators, history of chemotherapy and long-term use of either glucocorticoids or immunosuppressants. Severe thrombocytopenia meant a blood platelet count less than 20×109/L. The severity rating of the patient's acute condition at admission was based on APACHE II scores, the Charlson comorbidity indexes, the SOFA scores, and the Pitt bacteremia scores. Infectious sources were categorized as definite infection or colonization in other sites preceding BSI onset; otherwise the BSI was deemed primary. Septic shock definition followed sepsis 3.0.7
Bacterial detectionDrug susceptibility test was carried out with the broth microdilution method by a laboratory physician with analysis instruments (VITEK2 AST-GN16 French). Minimum inhibitory concentration (MIC) determination and interpretation were determined on a VITEK2 system, based on the Clinical and Laboratory Standards Institute (CLSI)8 recommendations. Carbapenem resistance meant imipenem and meropenem MIC≥4mg/L. It is noteworthy that resistance to tigecycline and colistin meant MIC>2mg/L, respectively, referring to the European Committee on Antimicrobial Susceptibility Testing (EUCAST, version 8.0, 2018).9
Statistical analysis: Data were analyzed using the statistical software package SPSS, version 22 (SPSS, Inc., Chicago, USA). Categorical variables in the two groups were compared with the Pearson χ2 or Fisher's exact test if cells had an expected frequency of 5 or fewer, and continuous variables with t-test for independent samples. 30-Day survival from bacteremia onset was plotted as Kaplan–Meier curves. The odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the strength of association. A p-value less than 0.05 was considered statistically significant.
ResultsA total of 69 patients who had CRKP bacteremia and received polymyxin B between Oct 1, 2015, and Mar 31, 2018 were retrieved. Forty cases were ultimately analyzed, and 29 cases were excluded. Refractory mixed infections occurred in 15 cases, including seven CRAB and four CRPA infections, three cases with MRSA, three cases with serious intracranial CRKP infections, and one case of invasive aspergillosis. More details can be seen in the flow chart (Fig. 1).
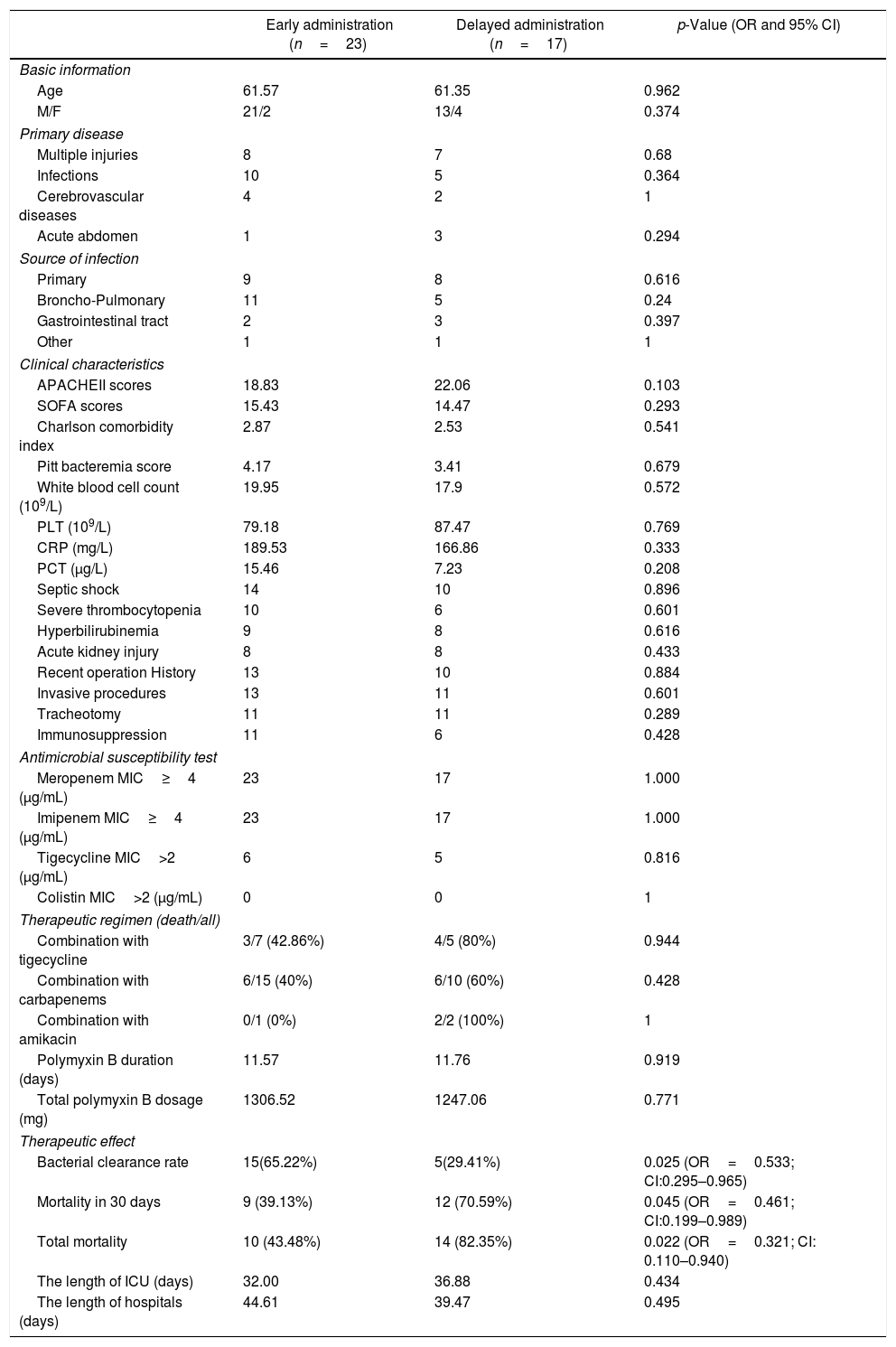
Table 1 reports the characteristics of patients with bacteremia due to CRKP and the variables associated with the timing of polymyxin B between early and delayed administration. The early use and delayed use groups had similar characteristics and great homogeneity. As a trauma center, complicated multiple injuries, as common primary diseases leading to ICU admission, accounted for 37.5%, the same as infectious diseases in our study. Age, sex and source of infection were similar in the two groups, and types of primary diseases were not significantly different. Broncho-pulmonary infection was considered the source of bacteremia in 16 patients and the gastrointestinal tract in five cases. Primary bloodstream infections, without clearly CRKP infectious or colonized sites, accounted for a large proportion (42.5%).
Characteristics of patients with bacteremia due to CRKP and variables associated with administration timing of polymyxin B between early and delayed administration.
| Early administration (n=23) | Delayed administration (n=17) | p-Value (OR and 95% CI) | |
|---|---|---|---|
| Basic information | |||
| Age | 61.57 | 61.35 | 0.962 |
| M/F | 21/2 | 13/4 | 0.374 |
| Primary disease | |||
| Multiple injuries | 8 | 7 | 0.68 |
| Infections | 10 | 5 | 0.364 |
| Cerebrovascular diseases | 4 | 2 | 1 |
| Acute abdomen | 1 | 3 | 0.294 |
| Source of infection | |||
| Primary | 9 | 8 | 0.616 |
| Broncho-Pulmonary | 11 | 5 | 0.24 |
| Gastrointestinal tract | 2 | 3 | 0.397 |
| Other | 1 | 1 | 1 |
| Clinical characteristics | |||
| APACHEII scores | 18.83 | 22.06 | 0.103 |
| SOFA scores | 15.43 | 14.47 | 0.293 |
| Charlson comorbidity index | 2.87 | 2.53 | 0.541 |
| Pitt bacteremia score | 4.17 | 3.41 | 0.679 |
| White blood cell count (109/L) | 19.95 | 17.9 | 0.572 |
| PLT (109/L) | 79.18 | 87.47 | 0.769 |
| CRP (mg/L) | 189.53 | 166.86 | 0.333 |
| PCT (μg/L) | 15.46 | 7.23 | 0.208 |
| Septic shock | 14 | 10 | 0.896 |
| Severe thrombocytopenia | 10 | 6 | 0.601 |
| Hyperbilirubinemia | 9 | 8 | 0.616 |
| Acute kidney injury | 8 | 8 | 0.433 |
| Recent operation History | 13 | 10 | 0.884 |
| Invasive procedures | 13 | 11 | 0.601 |
| Tracheotomy | 11 | 11 | 0.289 |
| Immunosuppression | 11 | 6 | 0.428 |
| Antimicrobial susceptibility test | |||
| Meropenem MIC≥4 (μg/mL) | 23 | 17 | 1.000 |
| Imipenem MIC≥4 (μg/mL) | 23 | 17 | 1.000 |
| Tigecycline MIC>2 (μg/mL) | 6 | 5 | 0.816 |
| Colistin MIC>2 (μg/mL) | 0 | 0 | 1 |
| Therapeutic regimen (death/all) | |||
| Combination with tigecycline | 3/7 (42.86%) | 4/5 (80%) | 0.944 |
| Combination with carbapenems | 6/15 (40%) | 6/10 (60%) | 0.428 |
| Combination with amikacin | 0/1 (0%) | 2/2 (100%) | 1 |
| Polymyxin B duration (days) | 11.57 | 11.76 | 0.919 |
| Total polymyxin B dosage (mg) | 1306.52 | 1247.06 | 0.771 |
| Therapeutic effect | |||
| Bacterial clearance rate | 15(65.22%) | 5(29.41%) | 0.025 (OR=0.533; CI:0.295–0.965) |
| Mortality in 30 days | 9 (39.13%) | 12 (70.59%) | 0.045 (OR=0.461; CI:0.199–0.989) |
| Total mortality | 10 (43.48%) | 14 (82.35%) | 0.022 (OR=0.321; CI: 0.110–0.940) |
| The length of ICU (days) | 32.00 | 36.88 | 0.434 |
| The length of hospitals (days) | 44.61 | 39.47 | 0.495 |
PLT, blood platelet; CRP, C-reactive protein; PCT, Procalcitonin; MIC, Minimal Inhibitory Concentration.
In terms of clinical characteristics, APACHE II scores (18.83 versus 22.06, p=0.103) and Charlson comorbidity indexes (2.39 versus 2.52, p=0.808) on admission into ICU were similar in both early and delayed use groups. Likewise, similar SOFA scores (15.43 versus 14.47, p=0.293) and Pitt bacteremia scores (4.17 versus 3.41, p=0.679) indicate that the patients had the same clinical condition when bloodstream infections were diagnosed. Laboratory parameters, including white blood cell count, blood platelet count (PLT), C-reactive protein (CRP), procalcitonin (PCT), and serum bilirubin were also similar in the two groups. Other conditions that may affect prognosis, such as acute kidney injury (AKI), invasive procedures, immunosuppression, and septic shock were not significantly different in the two groups, indicating homogeneity in the severity of infection.
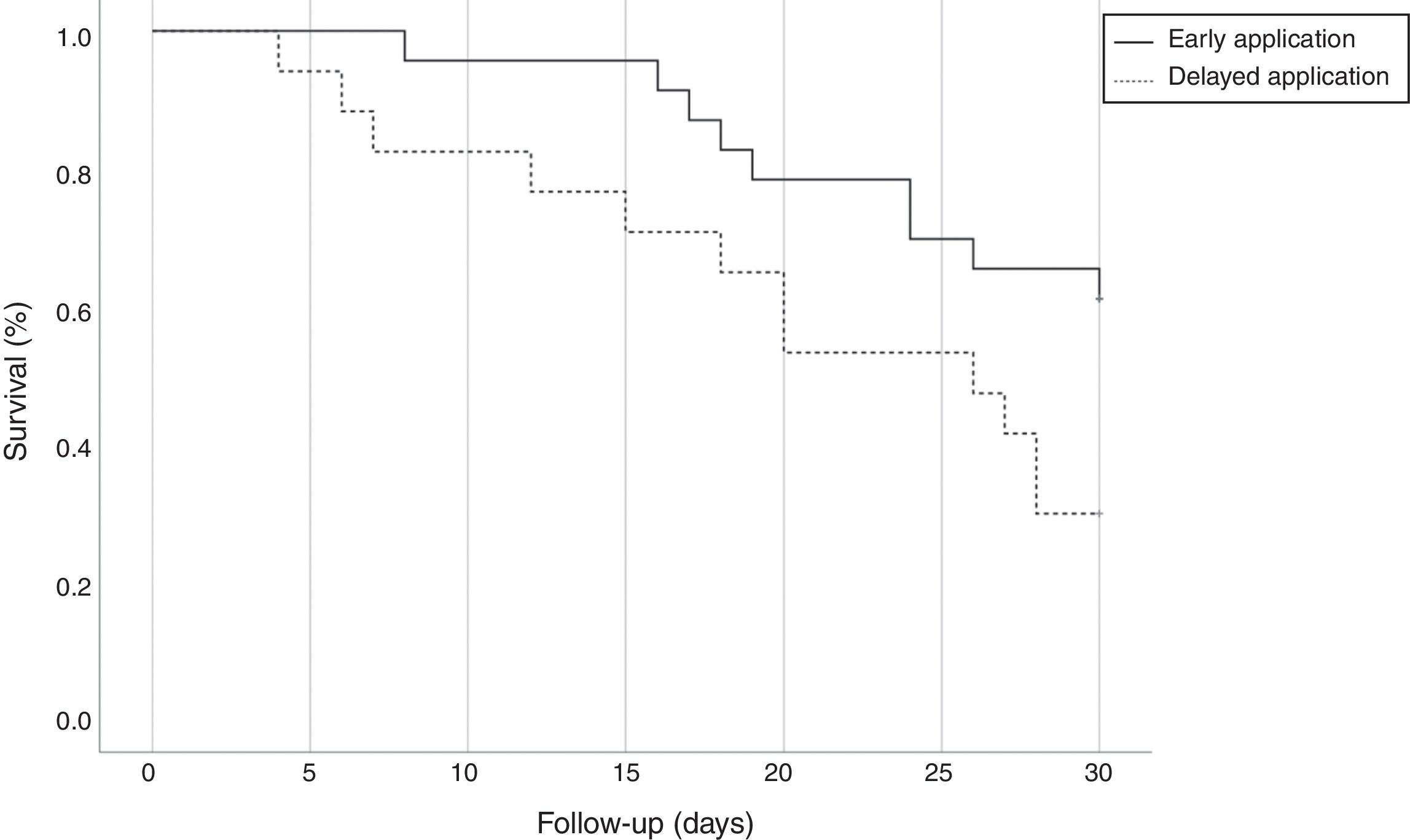
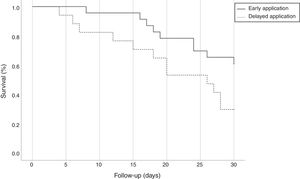
All isolates were resistant to meropenem and imipenem and sensitive to colistin. Nearly 27.5% of isolates were resistant to tigecycline. Although we used the most sensitive antibiotic, the overall lethality rate of CRKP bloodstream infections were still high. Twenty-four (60%) patients died, 10 (43.48%) patients with early administration of polymyxin B and 14 (82.35%) with delayed administration (p=0.022, OR=0.321; CI: 0.110–0.940). All patients accepted polymyxin B-based combination therapies. However, there was no difference between the three combinations with tigecycline, carbapenems, and amikacin. Compared with the delayed application, early use of polymyxin B showed greater bacterial clearance rate (65.22% versus 29.41%, p=0.025; OR=0.533; CI: 0.295–0.965) and reduced 30-day mortality (39.13% versus 70.59%, p=0.045; OR=0.461; CI: 0.199–0.989) and the overall mortality (43.48% versus 82.35%, p=0.022; OR=0.321; CI: 0.110–0.940). Fig. 2 shows the Kaplan–Meier curves of patients treated with early and delayed administration of polymyxin B (p=0.042). It should be pointed out that polymyxin B duration and total dosages in the two groups were similar (11.57 days versus 11.76 days, p=0.919; 1306.52mg versus 1247.06mg, p=0.711, respectively). Therefore, mortality was not correlated with longer treatment duration or higher dosage of polymyxin B. During the treatment, there were neither cases with detectable renal toxicity nor neurovirulence related to polymyxin B.
DiscussionIn our study, preemptive use of polymyxin B before blood culture results had better bacterial clearance and more favorable outcome in patients with BSI due to CRKP than delayed use of the drug.
Polymyxin B is an undisputed effective antibiotic choice for treating patients with CRKP bloodstream infections according to culture results. However, the limitations of traditional microbiological techniques in identifying bloodstream pathogens will delay treatment initiation of serious infections such as CRKP BSI. Furthermore, polymyxin B was only recently approved for entry into the Chinese market, and the price is exceedingly expensive. As a result, clinicians regard it as the last resort, preferring tigecycline or carbapenems, postponing the use of polymyxin B and unavoidably affecting prognosis. Gutiérrez-Gutiérrez et al. observed that appropriate therapy starting up to five days after infection was associated with decreased mortality (OR=0.44) in patients with BSIs due to CPE.5
Early appropriate therapy (starting up to two days after infection) can also reduce mortality (OR=0.84). We defined early use of polymyxin B as administering it before blood culture results, which is analogous to empirical antibiotic treatment. In contrast, the delayed group can be considered as having received targeted antibiotic therapy.10 However, there is some difference between early use in our study and empirical treatment, as half of the patients (57.5%) had primary infection sites, such as the broncho-pulmonary and gastrointestinal tract. Once the infectious symptoms occurred, BSI due to CRKP prompted early targeted therapies first. Nonetheless, most patients with primary BSIs had high risk factors, including invasive procedures, immunosuppression and history of carbapenems use.11,12 Patients in the delayed use group also had high risk of CRKP infections, but other antibiotic regimens were chose owing to the refusal of the patients’ relatives and the availability of alternative regimens. The total treatment duration and dosages of polymyxin B were similar in the two groups. Therefore, the advantages of early administration cannot be attributed to these factors. We speculate that early application of polymyxin B can reach an effective blood concentration as quickly as possible and truncating the duration of bacteremia, eventually reducing the death rate.
The impact of the underlying conditions in mortality of critical patients presenting with CRKP bacteremia are vital and could outweight the influence of antibiotic therapy. We tried our best to balance the characteristics of patients to avoid confounding primary diseases by employing strict eligibility criteria, such as excluding patients with terminal disease, intracranial CRKP infections, and high APACHE II scores (>30 points).
Although an optimal regimen is yet to be defined, most studies have recommended polymyxin B-based combinations in instead of monotherapy to reduce mortality.2,4,5,13 In a recent randomized controlled trial (RCT) by Mical Paul et al. polymyxin B combined with a carbapenem was not more effective than monotherapy in carbapenem-resistant Gram-negative bacterial infections. However, the main bacterium was CRAB rather than CRKP, and pneumonia and infections of other sites were included in this RCT, leading to great heterogeneity.14 In the study by Gutiérrez-Gutiérrez et al. polymyxin B was administered to 154 patients, half of them on monotherapy had a 30-day mortality of 54% (40/74), whereas those patients on polymyxin B-containing regimens had a 30-day mortality of 38% (28/74).5 Different combinations have been recommended as there are many possible treatment regimens. Carbapenem-containing regimens, as Daikos et al. reported, is among the most effective combinations.6 The survival rate was 52% with polymyxin B with carbapenems in our report, which was similar to the 50% synergy seen in vitro.15 In our study, polymyxin B was frequently combined with either a carbapenem, or tigecycline, or aminoglycosides. Two-thirds of patients received the combination of polymyxin B and a carbapenem, with a high mortality rate (61.11%). However, we cannot be able to draw any definitive conclusions because of the small sample sizes and nonsignificant statistical results. Our study has some limitations, such as the impacts of unmeasured variables and confounding factors due to the observational nature of the study.
ConclusionWe recommend early administration of polymyxin B-based combination therapy before blood culture results for patients with bloodstream infections due to CRKP. Irrespective of drug dosage and treatment duration, early use can effectively eliminate bacteria, improving 30-day and overall mortality.
Authors’ contributionsQiqiang Liang collected and analyzed the data and wrote the first draft of the manuscript. Zhijiang Xu assisted with the statistical analyses of the microorganisms. Man Huang reviewed and modified the draft of the manuscript.
FundingNo funding.
Conflicts of interestThe authors declare no conflicts of interest.
Xin XU and Tingting Gen assisted in selecting the clinical data.