Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly emerged virus that poses a great threat to human health because of high fatality rate.
MethodsTo develop sensitive and specific sero-diagnostic systems for SFTSV infections, monoclonal antibodies (MAbs) against recombinant SFTSV nucleocapsid (rSFTSV-N) protein were developed by immunizing BALB/C mice with rSFTSV-N protein and fusing the spleen cells with SP2/0 myeloma cells. Three hybridoma cell lines secreting MAbs against rSFTSV-N were obtained. MAb based IgG sandwich enzyme linked immunosorbent assay (ELISA) and IgM capture ELISA systems were established by using the newly developed MAbs. One hundred fifteen clinical suspected SFTS patients serum samples were used to evaluate the newly established systems by comparing with the total antibody detecting sandwich ELISA system and indirect ELISA systems.
ResultsThe MAbs based sandwich IgG ELISA was perfectly matched with that of the total antibody sandwich ELISA and the indirect IgG ELISA. IgM capture ELISA results perfectly matched with that of the total antibody sandwich ELISA while detecting eight additional positive samples missed by the indirect IgM ELISA.
ConclusionsThe MAbs against rSFTSV-N protein offer new tools for SFTSV studies and our newly developed MAb-based IgG and IgM capture ELISA systems would offer safe and useful tools for diagnosis of SFTS virus infections and epidemiological investigations.
Severe fever thrombocytopenia syndrome (SFTS) is an emerging, tick-borne infectious disease caused by SFTS virus, characterized by high fever, leukopenia, thrombocytopenia, and multiple organ dysfunctions, including the lung, liver, kidney and so forth.1-3 SFTSV is recognized as Phlebovirus genus in the Bunyaviridae family, with a mortality rate as high as 30% at early epidemic period.3,4 Since the first report of SFTS in China, the incidence of SFTS has expanded to at least 15 provinces in China. Other Asian countries such as South Korea, Japan and Vietnam have also identified this disease one after another, indicating that the epidemic area is extending.5-7 Therefore, due to the worldwide spread of SFTSV, high mortality rate, and the human communicable nature of the virus, the World Health Organization (WHO) has listed the SFTS virus as one of the most in need of attention pathogens.8 Nonetheless, to date, there are no available effective antiviral drugs or vaccines against SFTSV, and more diagnostic tools for detecting SFTSV are needed.9
Using clinical manifestations to diagnose SFTS is non-specific, since SFTS is difficult to distinguish from many other diseases with similar clinical features.6 Although virus isolation from blood of viremic patients is a reliable method to diagnose SFTSV infection, it is time-consuming and requires high security biocontainment facilities.10,11 There are several methods to detect SFTSV genome. Reverse transcription polymerase chain reaction (RT-PCR),11-13 quantitative real-time RT-PCR,11,14,15 and reverse transcription loop-mediated isothermal amplification assay (RT-LAMP)16-18 have been used for detecting the SFTSV genome. Studies have shown that these methods are only suitable for acute onset period of 1-6 days.1,10,19 Hence, additional methods for the diagnosis of SFTSV are essential.
SFTSV is a single-stranded, negative RNA virus with three segments, designated L, M, and S.20 The L segment encodes the RNA-dependent RNA polymerase (RaRp); the M segment encodes the glycoprotein precursor (GCP), including Gc and Gn glycoprotein; and the S segment encodes nucleocapsid protein (NP) and nonstructural protein (NSs) via using ambisense coding.21,22 NP is highly conserved and immunogenic in Bunyaviridae family. NP has been shown to play an important role in viral replication, transcription, and packaging of genomic RNA into ribonucleoprotein.23,24 Our previous study demonstrated that recombinant SFTSV nucleocapsid (rSFTSV-N) protein based indirect ELISA assay systems has been established to detect specific human IgG and IgM antibodies, respectively. However, rSFTSV-N protein based indirect IgM ELISA missed to detect several patients.25
In the present study, three monoclonal antibodies (MAbs: 5G12, 4A10, 1C3) against rSFTSV-N protein were successfully developed, and the MAb based IgG sandwich ELISA and IgM capture ELISA system were established. Serum samples, collected from clinically-suspected SFTS patients, were used to evaluate the newly established systems and results were compared with the total antibody detecting sandwich ELISA system and the indirect ELISA systems.
Materials and methodsSerum samplesTo evaluate the MAb based IgG sandwich ELISA and IgM capture ELISA system, 115 serum samples were collected from patients who recovered from suspected SFTS disease in Henan Province, China. The diagnostic criteria for suspected cases were as follows: relevant epidemiological history (i.e., working, living or traveling in the hilly, forest or mountain during SFTS epidemic season or being bitten by ticks within two weeks before disease onset), fever and other clinical manifestations with reduced peripheral blood platelet counts and leukopenia, following “Guideline for the prevention and treatment of fever with thrombocytopenia syndrome (2010 version)”.26
BALB/C mice immunizationThe rSFTSV-N protein expressed and purified as previously reported25 was emulsified with equal volumes of Freund's complete adjuvant (MP Biochemicals, CA, USA) and injected intraperitoneally into three 6-week old female BALB/c mice (Chongqing Tengxin Bill Experimental Animal Sales Co., LTD Chongqing, China), at a dose of 100 μg per injection. On day 14, all mice were boosted with rSFTSV-N protein in equal volumes of Freund's incomplete adjuvant (MP Biochemicals, CA, USA). Ten days after the second immunizations, blood samples were collected for antibody titer measurement via indirect ELISA. On day 28, 29, 30, mice were injected intraperitoneally with 100 μg/day rSFTSV-N protein for three times. On day 31, blood samples were collected and the spleen was taken out for cell fusion.
Preparation of monoclonal antibodies against rSFTSV-N proteinAfter immunization, the spleen cells were collected for cell fusion with SP2/0 myeloma cells (from the previous experiment)27 using polyethylene glycol (PEG 1500, Roche, Indiana, USA). The hybridoma cells were grown in a selective medium of hypoxanthine aminopterin - thymidine (HAT) (Gibco, NY, USA) for 10 days and then screened by indirect ELISA to select cells producing antibody against rSFTSV-N protein. The positive clones were diluted to establish single stable clones by limited dilution. In short, hybridoma cell suspension was diluted in growth medium (RPMI 1640, Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS) and inoculated to 96-well microplates with approximately 1 cell/well for 10 days before performing indirect ELISA to select clones secreting the desired antibody. Subsequently, the positive clones were transferred to culture flasks and propagated in growth media. At last, large-scale production of MAbs were done by propagating positive clones in Hybridoma-SFM medium (Gibco, NY, USA) and injected hybridoma cells intraperitoneally into mouse to produce aseptic fluid. MAb HiTrap protein G chromatograph kit (GE Healthcare, Uppsala, Sweden) was applied to purify MAbs.
Screening of MAbs by ELISAThe presence of antibodies in the supernatant of hybridoma cells culture media was assessed by ELISA according our previously published protocols27 with the following modification. The 96-well plates were processed as follows: coating of 50 ng/well rSFTSV-N protein at 4°C overnight, then blocking using PBS-T with 5% nonfat milk, at room temperature for 1h followed by adding hybridoma culture supernatant or adding pre-immunization and post-immunization mice serum samples for negative and positive controls, respectively, at 37°C for 1h; followed by the detection of bound IgG with horseradish-peroxidase-conjugated (HRP) goat anti-mouse IgG (American Qualex, San Clemente, CA) diluted 1:10,000 at 37°C for 1h. Then H2O2-ABTS [2,2’ azinobis (3-ethylbenzthiazolinesulfonic acid)] substrate was added and optical density (OD) value was recorded. All clones with OD value twice or higher than that of the negative control were considered positive.
Immunofluorescence analysisIndirect immunofluorescence test was applied to determine the reactivity of MAbs with SFTSV, SFTSV-infected Vero-E6 (from the previous experiment)27 were collected and centrifuged at 600 x g for five minutes, and then, the cell pellet was washed three times with PBS and spotted onto the Teflon-coated 8-multi-well glass slide (MP Biochemicals, CA, USA). After the slide was air dried, cells were fixed in cold acetone at 4°C for 10 min. After blocking with BlockAce for 30 min at room temperature, the cells were incubated with 15 μl culture media of the hybridoma cells in a wet chamber at 37°C for 1 h. Then, the slides were washed and air-dried once more, followed by detection using 15 μl of fluorescein isothiocyanate (FITC) conjugated goat anti-mouse IgG (Bethyl Laboratories Inc. Montgomery, USA) at a dilution of 1:50 to every test well and reacting in dark at 37°C for 1h. Finally, the slides were washed and sealed with mounting medium for fluorescence (VectorLaboratories, Inc.). The images were acquired using an OLYMPUS IX73 immunofluorescence microscope.
Western blottingWestern blot analysis was performed as described before.25 Briefly, protein molecular weight marker (PageRulerTM Prestained Protein Ladder, Thermo Scientific, Denmark) and the purified rSFTSV-N protein were separated in a 15% polyacrylamide gel. The separated proteins were transferred onto a polyvinylidene fluoride (PVDF) microporous membrane (Immobilon, Millipore, USA). The membrane was first blocked and then incubated at 4°C overnight or 37°C for 1 hour with the supernatant of hybridoma cells, followed by a secondary antibody of goat anti-mouse IgG (American Qualex, Califonia, USA, 1:1000 dilution). Protein bands were visualized using Metal Enhanced dimethyl aminobenzidine (DAB) Substrate Kits (Solarbio, China).
Detection of MAbs isotypesThe MAb isotype was determined by the SBA Clonotyping System-HRP kit according to the manufacturer's instructions (Southern Biotech, USA).
ELISA procedures for human serumTo evaluate MAbs for diagnosis of SFTSV infection, we established MAb-based IgG sandwich ELISA and IgM capture ELISA system for human sera. These ELISA systems that have common procedures are described here and the procedures specific for each assay will be described under each assay.
Ninety-six well Nunc immunoplates (Thermo Scientific, Denmark) were used with a sample volume of 100 μL/ well. The coating buffer was 0.01M PBS, pH 7.4, and the plate coating was done at 4°C overnight. After exposure to a specific reagent at each step of the system, except at the last step as described below, plates were washed three times with wash buffer (0.01 M PBS with 0.1% (vol/vol) Tween 20 (PBS-T)). PBS-T with 5% nonfat milk (Difco, Detroit, USA) was used to dilute all serum samples and reagents. In addition to substrate, all incubations were done at 37°C for 1 h. Besides, plates with 100 μL/well H2O2-ABTS substrate (Kirkegaatrd & Perry, Gaithersburg, MD) were incubated at 37°C for 30 min. A spectrophotometer was used to read the plates and record the OD values at 410 nm.
MAb-based IgG sandwich ELISAIn the MAb-based IgG sandwich ELISA, 96-well plates were processed as follows: coating of 50 ng/well of MAb overnight before blocking using PBS-T with 5% nonfat milk for 1h, followed by 50 ng/well rSFTSV-N protein, then human serum samples diluted at 1:1,000 in 5% nonfat milk in PBS-T were added, afterwards detection of bound IgG with HRP-goat anti-human IgG (American Qualex, Califonia, USA) diluted at 1:30,000, which was made visible after adding H2O2-ABTS substrate. OD values measured at 410 nm using a microplate spectrophotometer. Each serum specimen was tested twice and the average OD value was calculated. Each test used positive and negative control serum samples. The mean OD value of a sample more than twice the mean OD of the negative control serum was considered positive.
MAb-based IgM capture ELISAThe MAb-based IgM capture ELISA followed the steps below. Firstly, for each serum sample to be tested, four wells were coated with 1:500 dilution of anti-human IgM (Cappel, MP Biochemicals) before blocking using PBS-T with 5% nonfat milk for 1h. Secondly, 1:400 diluted human serum was added to the four wells. Afterwards two wells were added with 50 ng of rSFTSV-N protein (positive antigen) and other two wells were added with 50 ng of nonreactive protein (negative antigen). And then, 100 μL/well of purified MAb diluted at 1:10,000 was added to all four wells, after which a 1:10,000 diluted peroxidase conjugated anti-mouse IgG (American Qualex, Califonia, USA) was then added. Finally, the remaining steps for color development were the same as above.
Total antibody ELISA and indirect IgG, IgM ELISADetection of total antibody against SFTSV28 was done using a commercial ELISA kit (Xinlianxin Biomedical Technology CO., LTD, Wuxi, Jiangsu, China) following the manufacturer's instructions. The kit is a double-antigen sandwich enzyme-linked immunosorbent assay that detects total antibodies. This commercial kit has a sensitivity of 100% and specificity of 99.57% compared with virus neutralization testing.28 Indirect IgG and IgM ELISA were performed as previously described.25
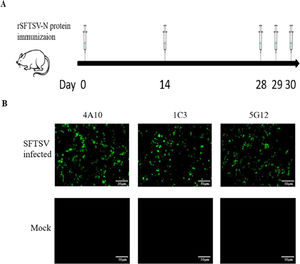
ResultPreparation of monoclonal antibodies against rSFTSV-N proteinBALB/c mice were immunized with rSFTSV-N protein five times with an immunization regimen as shown in Figure 1A. After subcloning and multiple rounds of indirect ELISA screening, three positive hybridoma clones producing MAbs against rSFTSV-N protein (designated as 5G12, 4A10, 1C3) were obtained.
Preparation of monoclonal antibodies against rSFTSV-N protein. (A) BALB/C mice immune schedule. (B) Immunofluorescence analysis to verify the specificity of MAbs. SFTSV infected and mock Vero-E6 cells reacted with three MAbs and then detected by FITC labeled anti-mouse IgG and observed under an immunofluorescence microscope.
Indirect immunofluorescence test showed strong fluorescence in Vero-E6 cells infected with SFTSV; no fluorescence was observed in mock Vero-E6 cells (Fig. 1B). Western blot assay showed that these three MAbs all reacted with rSFTSV-N protein (Fig. 2A). The classes of three MAbs were determined to be IgG2b and κ chain (Fig. 2B). These results confirmed that the three hybridoma cells secreted MAbs specific for SFTSTV.
Western blot ananysis and isotype detection of MAbs. (A) Western-blot analysis of MAbs against rSFTSV-N protein. a: MAb 4A10; b: MAb 1C3; c: MAb 5G12. Lane 1: protein marker; lane 2: purified rSFTSV-N protein. (B) The antibody subclasses of three MAbs. The subclasses of the 3 MAbs were determined to be IgG2b and κ chain using a mouse MAb isotyping kit.
To evaluate the possibility of using the newly developed MAbs for diagnosis, MAb-based IgG sandwich ELISA for human serum was established. Among the 115 human serum samples from SFTS suspected patients, 85 samples were IgG positive and 30 were IgG negative by the IgG sandwich ELISA system, and the three MAbs showed nearly uniform reactivity. These results perfectly matched the total antibody sandwich ELISA and the rSFTSV-N protein based indirect IgG ELISA results with a 100% concordance to these two ELISA systems (Tables 1 and 2).
Comparation of MAb-based IgG sandwich ELISA with total antibody sandwich ELISA.
| Total antibody sandwich ELISA | MAb-based IgG sandwich ELISA | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 85 | 0 | 85 |
| Negative | 0 | 30 | 30 |
| Total | 85 | 30 | 115 |
| Concordancea: 100% | |||
Comparation of MAb-based IgG sandwich ELISA with rSFTSV-N protein based indirect IgG ELISA.
| MAb-based IgG sandwich ELISA | rSFTSV-N-IgG indirect ELISA | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 85 | 0 | 85 |
| Negative | 0 | 30 | 30 |
| Total | 85 | 30 | 115 |
| Concordancea: 100% | |||
IgM capture ELISA for human serum was established using the MAb as detecting antibody. Among the 115 human serum samples from SFTS-suspected patients, 85 samples were IgM positive and 30 were IgM negative by the IgM capture ELISA system which perfectly matched the total antibody sandwich ELISA results with a 100% concordance with the total antibody sandwich ELISA system (Table 3), and similarly, the three MAbs showed nearly uniform reactivity in this method.
Comparation of MAb-based IgM capture ELISA with total antibody sandwich ELISA.
| Total antibody sandwich ELISA | MAb-based IgM capture ELISA | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 85 | 0 | 85 |
| Negative | 0 | 30 | 30 |
| Total | 85 | 30 | 115 |
| Concordancea: 100% | |||
Compared with the rSFTSV-N protein based indirect IgM ELISA, among the 85 positive samples detected by IgM capture ELISA, indirect IgM ELISA detected 77 positive, missing eight positive samples. The concordance of the two methods was 93.04% (Table 4).
Comparation of MAb-based IgG sandwich ELISA with rSFTSV-N protein based indirect IgM ELISA.
| MAb-based IgM capture ELISA | rSFTSV-N-IgM indirect ELISA | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 77 | 8 | 85 |
| Negative | 0 | 30 | 30 |
| Total | 77 | 38 | 115 |
| Concordancea: 93.04% | |||
Severe fever with thrombocytopenia syndrome is an acute viral infection mainly transmitted through tick bites, and person-to-person transmission has been reported.29 SFTSV has generated great concern as SFTS cases and related deaths are on the rise with high fatality rates ranging from 16.4% to 30%.30 For the diagnosis of SFTS, clinical manifestations of SFTS are non-specific, and as the direct evidence of SFTS infection, virus isolation from SFTSV-infected patients is time-consuming and requires highly safe biological protection facilities.11,19 Therefore, safe methods with high sensitivity and specificity for the diagnosis of SFTSV are indispensable.
Due to high immunogenicity and abundance of NP in viral particles and infected cells, the recombinant NP is appropriate for using as a diagnostic antigen.10,23,25 Because of the high purity and strong specificity, monoclonal antibodies can improve the sensitivity and specificity of various serological methods for detecting antigens.27,31,32 MAbs against rSFTSV-NP for the diagnosis of SFTS have been documented for antigen detection and bovine serum antibody detection,33-35 but there is no report for application in detecting human antibodies. Detecting IgG or IgM antibodies in sera is a well-known method for confirming infections, and studies have shown that IgM antibody detection is suitable for the early diagnosis of infection.12,36,37
Using the immunization schedule shown in Figure 1A, we used purified rSFTSV-N protein to immunize mice, and extracted the spleen cells for cell fusion with SP2/0 to prepare hybridoma cell lines secreting anti-rSFTSV-N protein MAbs. Three hybridoma cell lines secreting MAbs, named 5G12, 4A10, 1C3, with strong and specific reactivity to SFTSV were obtained. The MAbs reacted with the rSFTSV-N protein in indirect ELISA, immunofluorescence assay (Fig. 1B) and Western blot (Fig. 2A). These MAbs against SFTSV offer useful tools for the study of SFTSV.
MAb-based IgG sandwich ELISA and IgM capture ELISA to detect human serum antibodies against SFTSV were established and compared with the commercial total antibody detecting sandwich ELISA system, indirect IgG and indirect IgM systems, respectively. Previous study has shown that the sensitivity of this commercial kit is 100% and the specificity is 99.57%.28 Serum samples of 115 suspected SFTSV patients were evaluated, and the concordance rates of MAb-based IgG sandwich ELISA or IgM capture ELISA with a total antibody detection sandwich ELISA and the indirect IgG ELISA were all 100%, indicating that the newly developed MAb based ELISA systems are useful methods for detecting SFTSV antibodies (Tables 1, 2 and 3).
The IgM capture ELISA detected eight additional positive samples missed by the indirect IgM ELISA, probably because the IgM capture ELISA eliminated the competed binding of IgG to antigen with the IgM.25,38-40 We have successfully developed two in-house MAbs-based ELISA systems, which do not require high-level microbiological safety facilities to prepare and use virus hence safe methods. The new systems detect IgG or IgM antibody separately, thereby they can distinguish previous from recent infection, thus provide more useful tools for SFTSV-infection diagnosis and epidemiological investigations.
There are several limitations of the current study. First, the major limitation of the study is the lack of tests like PCR test for confirmation of suspected patients. Second, because of the small amount of remaining serum, neutralization testing was not done. Third, the newly developed MAb-based IgG sandwich ELISA and IgM capture ELISA need to be evaluated with increased number of serum samples from different places.
ConclusionMAbs against rSFTSV-N protein were successfully developed and used in MAb-based IgG sandwich ELISA and IgM capture ELISA systems for human serum. The two systems are safe, convenient, and affordable for the diagnosis of SFTS.
Authors’ contributionsM.Z., F.Y., B.Y., K.M.: Study design; M.Z., L.Y., X.H.: Laboratory experiments; M.Z., Y.D., L.Z., B.X.: Data analysis; M.Z., F.Y.: Writing of the manuscript. All authors have read and agreed to the published the final manuscript.
FundingThis work was supported by the National Natural Science Foundation of China ((2017) 81760605); the doctor's funding of Guizhou Provincial People's Hospital GSYSBS [2016]01.
Ethics approval and consent to participateThis study was approved by the Ethical Committee of the Henan Provincial Center for Disease Control and Prevention (2016-KY-002-02). All experiments are carried out in compliance with approved guidelines and regulations. Part of this experiment used the remaining samples from the previous experiment. All serum sample providers have written informed consent to use their serum samples for research purposes.
We would like to thank the members of the Central Laboratory of Pulmonary Immunological Disease Research Centre, Chinese Health Ministry for their technical assistance and helpful discussions. We also acknowledged the School of Medicine, Guizhou University, for the training and support.