BACTEC 460 has now been phased out, so the search for an alternative is imperative. We have determined the activity of standard anti-tuberculosis drugs against intramacrophage Mycobacterium tuberculosis, in vitro, by using BACTEC 460 and MGIT 960 methods. The minimum inhibitory concentrations of isoniazid, rifampicin, ethambutol and streptomycin against intracellular M. tuberculosis H37Rv were found to be 0.2, 0.8, 8.0, and 5.0μg/mL, respectively, by both methods. These results show a significant (p<0.001) concordance between minimum inhibitory concentrations obtained by these two different methods. MGIT 960 system uses a robust florescence quenching-based oxygen sensor, requires no radioisotope, is safe, and relatively easy to operate. Apparently, this is the first report wherein MGIT 960 has been validated for anti-tubercular susceptibility testing against intracellular M. tuberculosis H37Rv. Our preliminary data thus clearly demonstrate that the MGIT 960 method can be considered as a promising alternative to BACTEC 460 method.
Tuberculosis (TB) is a global pandemic and a re-emerging bacterial infectious disease caused by obligate human pathogen Mycobacterium tuberculosis. It affects one third of the human population.1 There is an estimated 2.5 billion people infected with TB worldwide with 8.7 million (range 8.3–9.0 million) new cases, and an annual mortality of approximately 1.0 million (range 0.84–1.1 million) people, and an additional mortality of 0.43 million (range 0.40–0.46 million) among human immunodeficiency virus (HIV)-infected people.2 TB kills one individual in every 18s and infects an individual every second.3 Despite the availability of effective drugs to treat TB patients, we have failed to stop the menace of this deadly disease, which has worsened due to emergence of both multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant (XDR-TB).4–6 The increase in the incidence of drug resistant TB in HIV infected individuals is a leading cause of death, and is further worsening the TB situation worldwide.
The major phenomenon in the development of TB in humans is considered to be an interaction between macrophages and the mycobacteria. Various standardized methods are commonly used to study the susceptibility to drugs of intracellular mycobacteria such as plating, micro plate alamar blue assay and BACTEC 460.7–9
Both plating (conventional) method and BACTEC 460 (Becton, Dickinson, USA) are considered as gold-standard for measuring the growth of intracellular mycobacteria in macrophages.7–11 Plating method requires longer period of incubation (almost three weeks) and the results are compromised by cross contamination. However BACTEC 460 system has a shorter period of incubation, is highly sensitive and reproducible, but it also has certain limitations. Firstly, it involves the use of radioisotopes and requires special attention during disposal.12 Secondly, it is labor intensive, semi-automated, needs considerable hands on time.12 Moreover, BACTEC 460 has been discontinued by manufacturer due to radioactivity related safety issues.
Therefore, there is an urgent need to develop/standardize new methods, which are free of such limitations. Several evaluations concerning antimicrobial susceptibility testing using MGIT (mycobacterial growth indicator tube; Becton Dickinson, USA) medium have already been reported.13–16 Its performance has been found to be equivalent to radiometric BACTEC 460, plating and other methods.16 In this study, we have tried to standardize the method for studying the effect of isoniazid (INH), rifampin (RIF), ethambutol (EMB) and streptomycin (STP) against intramacrophage M. tuberculosis, in vitro using MGIT 960, which is a fully automated, non-radioactive (use a fluorescence quenching based oxygen sensor), and non-invasive system. In this MGIT 960 system, tubes are monitored automatically within in-built incubation system. Every tube is monitored hourly and specific algorithms that determine the positivity of tubes are drawn automatically.13–16
Materials and methodsINH, RIF, EMB and STP were purchased from Becton Dickinson, USA. These drugs were used to test the susceptibilities of the mycobacteria using BACTEC 460 and MGIT 960 system. The solutions of these standard drugs were prepared in sterile water as per manufacturers’ instructions.
M. tuberculosis H37Rv was obtained from Tuberculosis Research Centre (TRC), Chennai, India. The organisms were grown in complete 7H9 broth (Difco, USA) supplemented with 1% glycerol, 0.05% Tween-80 (Sigma–Aldrich, USA) and 10% Middlebrook OADC enrichment (Difco). Log phase cultures of mycobacteria were centrifuged and washed thrice with saline. After washing, pellets were re-suspended in saline and allowed to stand at room temperature for 10min. The mycobacterial suspension was adjusted to 1 McFarland standard (3×108cells/mL) with DMEM containing 1% fetal calf serum for inoculation. The purity of culture was checked by Ziehl–Neelsen staining (Hi-Media, India).
Swiss mice (male/female; 18–20g) were obtained from the central animal facility of the institute and maintained in 12-h light/dark cycle at 37°C, with food and water provided ad libitum. All studies were carried out in accordance with the guidelines for care and use of animals in scientific research, Indian National Science Academy, New Delhi as adapted and promulgated by the institutional animal ethics committee.
Thioglycolate elicited mouse peritoneal macrophages (PMs) exudate cells were isolated as described earlier.17 Briefly, the cells were washed and finally suspended in antibiotic-free DMEM (PAA lab, Austria) containing 2mM l-glutamine (PAA lab), 0.01M HEPES (HiMedia, India) and 1×10−4M 2-mercaptoethanol, supplemented with 10% FBS (PAA Lab; CDMEM). The cell pellets were seeded in 24-well sterile tissue culture plates with 1×106cells/well in a total volume of 2mL per well. The cells were incubated overnight at 37°C in 5% CO2 incubator for adherence, and the non-adherent cells were washed-off with warm Hank's balanced salt solution (HBSS). Purity of PMs was checked as per morphologic, phagocytic and non-specific esterase staining criteria, and viability was judged by trypan blue dye exclusion method.17
Adherent layer of PMs was infected with M. tuberculosis H37Rv suspension containing 107 bacteria per well (multiplicity of infection 10:1) and the phagocytosis was allowed for 4h at 37°C in 5% CO2 atmosphere. All the extracellular mycobacteria were washed by three changes of warm HBSS. The contents of the wells were replaced with fresh DMEM with or without drugs and incubated in (37°C, 5% CO2) incubator. Supernatant was removed after specific periods (four days and seven days) of incubation, washed three times with HBSS and PMs were lysed by addition of 0.4mL of 0.25% sodium dodedcyl sulphate and 1.1mL of Middle Brook 7H9 media. After 10min of incubation, 0.5mL of 20% bovine serum albumin was added and further incubated for 10min. The cell suspension was vortexed, sonicated and drawn up and down through a needle so as to get homogeneous single cell suspension.
Antibiotic susceptibility was performed by radiometric BACTEC 460 TB system as per standard protocol.15 Different dilutions of macrophage lysates (as prepared above) were inoculated in BACTEC 12 B vials in triplicate and incubated at 37°C in 5% CO2 atmosphere. Results were read every 24h in BACTEC 460 TB system. Appropriate positive and negative controls were also taken. Growth index (GI) determination was done daily under aerobic mode in BACTEC 460 TB system until 1:100 controls reading reached the value of 30.
The antibiotic susceptibility was also performed with MGIT 960 in accordance with the standard procedure.18 MGIT 960 system uses oxygen quenching fluorescent sensor in conjunction with software algorithms to determine the “positive” tubes. MGIT 960 system monitors the vials for oxygen utilization, which result in an increase in fluorescence as an indicator of growth. Different dilutions of macrophage lysates (as prepared above) were inoculated in MGIT 960 tubes in triplicate. Bacterial growth is determined in terms of growth units (GU). Appropriate positive and negative controls were included. GU was monitored in MGIT 960 system until 1:100 control reading reached the threshold value.
Both the methods were employed to determine MICs of standard drugs. In BACTEC 460, the MIC is defined as the lowest concentration of drug at which the increase in GI was equal to or less than that of control (1:100).19,20 In MGIT 960, the MIC is defined as the lowest dilution that gives negative results by automated reading in drug-containing tubes when the 1:100 control vials turned positive.18
All results were analyzed using Sigma plot Statistical Software 11.0. The values were expressed as mean±SD of three separate experiments, run in triplicate. Multiple groups were analyzed by one-way ANOVA followed by Turkey's multiple comparison tests. p<0.05 was considered as statistically significant.
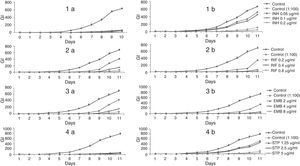
ResultsIn the present study, MICs of RIF, INH, EMB, and STP were determined against intramacrophage M. tuberculosis H37Rv, in vitro. MICs of INH, RIF, EMB, and STP against intracellular M. tuberculosis H37Rv were found to be 0.2, 0.8, 8.0, and 5.0μg/mL respectively, by both BACTEC 460 and MGIT 960 methods. Fig. 1 show that INH was also effective (p<0.001) in killing the M. tuberculosis H37Rv-infected PMs at 0.05, 0.1 and 0.2μg/mL concentrations using BACTEC 460 at 4th day and 7th day. Similarly, Fig. 1 also summarizes the effect of RIF, EMB, STP against M. tuberculosis H37Rv-infected PMs. RIF was found to be effective at 0.2, 0.4 and 0.8μg/mL concentrations using BACTEC 460 at 4th day and 7th day (Fig. 1). EMB was found to be effective at 2.0, 4.0 and 8.0μg/mL concentrations using BACTEC 460 at 4th day and 7th day (Fig. 1). In the same way, STP was effective at 1.25, 2.50, 5.0μg/mL against intracellular M. tuberculosis (Fig. 1). The results were found to be highly statistically significant (p<0.001). In the same way, various concentrations of INH, RIF, EMB and STP showed almost similar effect in killing the intracellular M. tuberculosis H37Rv using MGIT 960 at 4th day and 7th day. These results were also found to be highly statistically significant (p<0.001) at all the three different concentrations. Various growth stages of mycobacteria using epicenter software are shown in Fig. 2.
Typical radiometric data illustrating the MIC level of Isoniazid (INH), Rifampin (RIF), Ehambutol (EMB) and Streptomycin (STP); at 4 day (a) and 7 day (b) interval against the drug susceptible M. tuberculosis strain. PMs (2×106) were infected in culture at a bacterium to macrophage ratio (10:1). Infected PMs without drug treatment were used as control (1:100). Error bars represent the standard deviation from mean. Values are mean±SD of three separate experiments, run in triplicate. Statistical analysis was performed by ANOVA and Tukey's multiple comparison test. The growth was monitored radiometrically in 7H12 broth with the BACTEC 460 TB system.
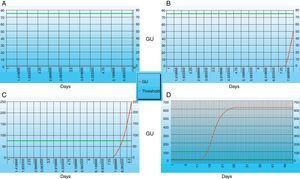
MGIT growth and detection plot. Red line indicates growth units of inoculated culture and green line indicates threshold value (minimum value of growth units at which our culture sample become positive). (A) No growth; (B) increase in growth; (C) growth units crosses the threshold value (our culture sample become positive); (D) stationary phase (no subsequent increase in growth). All the images are obtained through epicenter software.
Plating method is a laborious procedure and requires long period of incubation. Since 1994, BACTEC 460 has been widely employed for determining the anti-tubercular activity of various drugs against intracellular M. tuberculosis H37Rv, and it gives results within 5–6 days. But due to increasing concerns about radioactivity and its disposal, it has been discontinued/phased out by the manufacturer. Therefore, there is an urgent need to standardize a method to overcome these shortcomings. Although, there is no impendent to M. tuberculosis H37Rv susceptibility testing with non-automated liquid media, only automated system like MGIT 960 has the potential to replace the semi-automated BACTEC 460 TB system. Among all the automated systems, MGIT 960 has shown good sensitivity and reliability for screening the anti-tubercular activity of drugs, in vitro. Here, we have tested the utility, reliability and reproducibility of MGIT 960 to determine the intracellular activity of four anti-TB drugs RIF, INH, EMB, and STP against M. tuberculosis H37Rv, in vitro. We have evaluated the BACTEC 460 TB against MGIT 960, and a good correlation was observed between the two methods for all the drugs tested in this model. Time needed for test completion was found to be almost similar for these methods. The MGIT 960 system has several advantages over the widely used BACTEC 460 system. It is less labor intensive and no radioactive material is involved; moreover, it is safer for laboratory personnel. Although MGIT 960 has been used for anti-tubercular testing, this is the first report of use of MGIT 960 for anti-tubercular susceptibility testing against intracellular M. tuberculosis H37Rv. MGIT 960 is easy, precise and less hazardous for determination of MICs in intracellular model. Thus MGIT 960 system has a good potential and it appears to be a valid alternative to BACTEC 460 radiometric system in the determination of antibacterial activity of various drugs against intracellular TB bacilli in macrophage model. Further studies may be performed using different M. tuberculosis isolates against first-line and second-line anti-TB drugs to evaluate MGIT 960 utility in determination of intracellular MICs. MGIT 960 system appears to be a good alternative/addition to existing methods for carrying out susceptibility testing against intracellular M. tuberculosis.
Conflicts of interestThe authors declare no conflicts of interest.
We are grateful to Prof. K. K. Bhutani, Officiating Director, National Institute of Pharmaceutical Education and Research for his help and encouragement. We thank Mr. Vijay Kumar Misra for technical assistance. Mr. Amit Goyal is grateful to CSIR, New Delhi, India for the award of a Senior Research Fellowship. We also acknowledge European Union for the financial support in part, under Indo-EU seventh framework program.