This study aimed to characterize Staphylococcus aureus isolates from bloodstream infections in patients attending a teaching hospital, between 2011 and 2015.
MethodsThe minimum inhibitory concentration for daptomycin, linezolid, oxacillin, teicoplanin, vancomycin, and trimethoprim/sulfamethoxazole was accessed by broth microdilution. SCCmec type and clonal profile were determined by molecular tests. Vancomycin heteroresistance was evaluated using screening tests and by population analysis profile/area under the curve.
ResultsAmong 200 S. aureus isolates, 55 (27.5%) were MRSA, carrying SCCmec II (45.5%) or IV (54.5%). The most frequent MRSA lineages were USA100 (ST5-II) (45.5%) and USA800 (ST5-IV) (30.9%). Six isolates were confirmed as vancomycin heteroresistant, showing area under the curve ratio 1.1, 1.2 or 1.3 (four USA100, one USA800 and one USA1100 isolates).
ConclusionsDaptomycin and vancomycin non-susceptible MRSA clonal lineages were found in bloodstream infections over five years, highlighting the importance of continuous surveillance of multiresistant bacteria in hospitals.
Staphylococcus aureus bloodstream infection (BSI) is one of the most important causes of morbidity and mortality in hospitals, and its treatment has become more challenging over the years.1 Vancomycin is the main choice for treating methicillin-resistant S. aureus (MRSA) BSI. Due to constant change of epidemic clones, showing different patterns of resistance in the hospital settings, old antimicrobials, such as trimethoprim/sulfamethoxazole (TMP/STX) may be an alternative in the treatment of invasive infections.1 Daptomycin has been efficacious in the treatment of BSI and endocarditis by S. aureus.2 However, there is lack of data regarding rates of resistance to this antimicrobial in S. aureus isolates from BSI in Brazil.
In Brazil and in other developing countries, data about antimicrobial resistance rates and genotypic characteristics of S. aureus isolates from BSI are still scarce and performed with very few isolates.3 In a previous study, we analyzed clinical and microbiological characteristics of S. aureus from bloodstream infections (BSI) in a Brazilian teaching hospital, between 2011 and 2013, focusing on their susceptibility to vancomycin.4 Here, we continued analyzing S. aureus isolates from BSI in the same hospital in order to provide more conclusive data about antimicrobial resistance profiles and clonal lineages to help the infection control and prevention team cope with this type of staphylococcal infection.
This study was performed at the University Hospital “Clementino Fraga Filho” (HUCFF), a 490-bed university teaching hospital located in Rio de Janeiro, Brazil. In the last years at HUCFF, S. aureus has been responsible for about 15% of BSI. The study was approved by Human Research Ethics Committee of the HUCFF (No. 40652714.0.0000.5257) and evaluated consecutive BSI S. aureus isolates from adults, between February 2011 and December 2015. Only the first isolate of a BSI episode was analyzed. Clinical data from patients with hVISA BSI were collected in a confidential manner, ensuring the privacy of the subjects involved.
Previous bacterial identification was carried out by the automated VITEK2® system (BioMerieux, Durham, USA) and confirmed using classical tests.5 Susceptibility to methicillin was determined by disk diffusion test.6 MICs were determined by broth microdilution (BMD) for daptomycin, linezolid, oxacillin, teicoplanin, vancomycin, and TMP/STX (Sigma-Aldrich Chemical Company, St Louis, USA).6 The mecA gene detection and SCCmec typing were performed as previously described.7 Pulsed-field gel electrophoresis (PFGE)8 and multilocus sequence typing (MLST) were performed for MRSA isolates.9
MRSA isolates with vancomycin MIC=2mg/L were screened for hVISA, using BHI agar with 4mg/L of vancomycin and 16g/L of pancreatic digest of casein (Merck, Darmstadt, Germany) (BHI4ca),10 and Etest® (bioMerieux) macromethod. S. aureus ATCC 29213 and Mu3 (hVISA) were used as control strains. MRSA isolates displaying at least one positive screening test result were subjected to population analysis profile/area under the curve (PAP-AUC).11 Isolates were identified as hVISA if the AUC ratio was ≥0.9 and ≤1.3.
Two-tailed Fisher's exact test was used to calculate p-values. A p-value ≤0.05 was considered statistically significant.
A total of 200 S. aureus isolates were included in the study. Nine individuals had two distinct episodes of BSI (n=191 patients). Fifty-five (27.5%) isolates were MRSA, being 25 (45.5%) SCCmec II and 30 (54.5%) SCCmec IV. The MIC50 and resistance or non-susceptibility rates to the antimicrobials are shown in Table 1. Six isolates were VISA (4 MRSA and 2 MSSA). Forty-seven (23.5%) isolates were non-susceptible to daptomycin (MIC >1mg/L), being 20 MRSA and 27 MSSA. MRSA isolates had higher rates of vancomycin resistance and daptomycin non-susceptibility then MSSA isolates (p=0.02 and 0.04, respectively) (data not shown).
MIC50 and percentual of antimicrobial resistance among 200 Staphylococcus aureus isolates from bloodstream infection for five years (2011–2015) in a teaching hospital in Rio de Janeiro according clonal lineages.
| S. aureusClonality/ST (n) | Oxacillin | Vancomycin | Daptomycin | Teicoplanin | TMP/STX | Linezolid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 | n (%) R | MIC50 | n (%) R | MIC50 | n (%) R | MIC50 | n (%) R | MIC50 | R (%) | MIC50 | n (%) R | |
| MSSA (145) | 0.5 | 0 (0) | 1 | 2 (1.4) | 1 | 27 (18.6) | 0.25 | 0 (0) | ≤0.125/2.3 | 0 (0) | 2 | 0 (0) |
| MRSA (55) | 64 | 55 (100) | 1 | 4 (7.3) | 1 | 20 (36.4) | 0.5 | 0 (0) | ≤0.125/2.3 | 0 (0) | 2 | 0 (0) |
| USA100/ST5 (25) | 256 | 25 (100) | 2 | 3 (12) | 1 | 11 (44.0) | 0.5 | 0 (0) | ≤0.125/2.3 | 0 (0) | 1 | 0 (0) |
| USA800/ST5 (17) | 16 | 17 (100) | 1 | 1 (5.9) | 1 | 4 (23.5) | 0.5 | 0 (0) | ≤0.125/2.3 | 0 (0) | 2 | 0 (0) |
| USA1100/ST30 (10) | 4 | 10 (100) | 1 | 0 (0) | 1 | 2 (20.0) | ≤0.125 | 0 (0) | ≤0.125/2.3 | 0 (0) | 2 | 0 (0) |
| USA400/ST1 (3) | 16 | 3 (100) | 2 | 0 (0) | 1 | 1 (33.3) | 0.5 | 0 (0) | ≤0.125/2.3 | 0 (0) | 1 | 0 (0) |
ST, sequence type; TMP/STX, trimethoprim/sulfamethoxazole; MIC, minimum inhibitory concentration; R, resistance; MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus. Resistance breakpoints6 – oxacillin: ≥4μg/mL, vancomycin: ≥4μg/mL, daptomycin: >1μg/mL, teicoplanin: >1μg/mL, TMP/STX: ≥4/76μg/mL, linezolid: ≥4μg/mL.
A total of 36 (18%) S. aureus isolates showed vancomycin MIC=2mg/L, 19 (53%) MRSA, which were then tested for vancomycin heteroresistance by screening tests. Thirteen isolates were positive for these tests and six of them were confirmed as hVISA, showing an AUC ratio 1.1, 1.2 or 1.3.
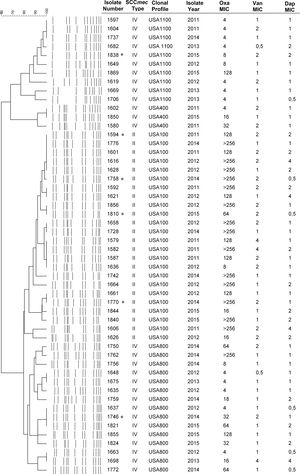
The MRSA lineages found were USA100 (ST5-II) (45.5%), followed by USA800 (ST5-IV) (30.9%), USA1100 (ST30-IV) (18.2%), USA400 (ST1-IV) (5.4%) (Fig. 1). The Brazilian clone (ST239-III) was not found. The most prevalent lineage USA100 tended to present multidrug resistance, with high MIC values for oxacillin, vancomycin, and daptomycin. Notably, most of the daptomycin non-susceptible MRSA isolates (65%) belonged to this lineage. Moreover, three VISA isolates were USA100.
Bloodstream infections caused by MRSA was associated with higher mortality rates than those caused by sensitive isolates.1 In this scenario, surveillance of antimicrobials resistance plays a major role by providing important data for empirical treatment decision. As data on antimicrobial susceptibility in Brazil is scarce, especially for daptomycin and TMP/STX, the present study highlighted an unexpected high vancomycin and daptomycin MICs with low TMP/TSX MICs among S. aureus isolates from BSI in a Brazilian hospital over five years.
Daptomycin has been frequently used in persistent bacteremia in cases with MIC values for vancomycin >1mg/L.12 However, in our study a substantial number of isolates were non-susceptible to daptomycin. Interestingly, most of the daptomycin non-susceptible MRSA isolates were associated with the USA100 (ST5-II), the same lineage that presented six vancomycin non-susceptible isolates (3 hVISA and 3 VISA). Although there is no daptomycin resistance surveillance in Brazil, which makes comparison with our results impossible, a high non-susceptibility, as the one found in the hospital of study, may not represent most of the Brazilian hospitals. It should be noted that daptomycin non-susceptible isolates belonging to the USA100 lineage have already been reported in Brazil.3 In addition, there was an association between the MRSA phenotype and resistance to vancomycin and daptomycin (p≤0.05). This result is of concern once daptomycin is an important alternative for the treatment of BSI by MRSA, VISA or hVISA, phenotypes that were all found in the present study.
In this study, we found 10 USA1100 (ST30-IV) isolates recovered from BSI infections. Schuenck et al.13 had already described USA1100 causing invasive infections in patients hospitalized at the same hospital. Notably, this lineage is commonly observed in individuals at the community. Thus, we hypothesized that isolates of USA1100 lineage were introduced to the hospital analyzed through the admission of colonized patients, as we recently evidenced in this same hospital.14
Chamon and colleagues,15 in a study conducted at the same hospital, found that USA400 (ST1-IV) and USA800 (ST5-IV) were the most common lineages between 2008 and 2009, also showing the presence of one USA100 (ST5-II) isolate among BSI MRSA isolates. Therefore, there has been a change in the epidemiology of BSI in our hospital, highlighting clonal profiles that were infrequent in the past. Although the present study involves only one Brazilian hospital, the results reflect the decline in the prevalence of the BEC lineage and its replacement by other international clones, leading to significant differences in antimicrobial susceptibility patterns.
In this study, non-susceptibility phenotypes to daptomycin and vancomycin were observed among S. aureus isolates from BSI over five years at a Brazilian teaching hospital. Considering the change in the epidemiology of S. aureus infections in recent years in Brazil, these results seem to be related with MRSA clonal lineages dissemination in this institution, reinforcing that surveillance and spread control of multiresistant bacteria should be continuously monitored in health institutions worldwide.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by Brazilian grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.