Despite the success of Antiretroviral Therapy (ART) in preventing HIV-1-associated clinical progression to AIDS, it is unable to eliminate the viral reservoirs and eradicate the HIV-1 infection. Therapeutic vaccination is an alternative approach to alter the HIV-1 infection course. It can induce effective HIV-1-specific immunity to control viremia and eliminate the need for lifelong ART. Immunological data from spontaneous HIV-1 controllers have shown that cross-reactive T-cell responses are the key immune mechanism in HIV-1 control. Directing these responses toward preferred HIV-1 epitopes is a promising strategy in therapeutic vaccine settings. Designing novel immunogens based on the HIV-1 conserved regions containing a wide range of critical T- and B-cell epitopes of the main viral antigens (conserved multiepitope approaches) supplies broad coverage of global diversity in HIV-1 strains and Human Leukocyte Antigen (HLA) alleles. It can also prevent immune induction to undesirable decoy epitopes theoretically. The efficacy of different novel HIV-1 immunogens based on the conserved and/or functional protective site of HIV-1 proteome has been evaluated in multiple clinical trials. Most of these immunogens were generally safe and able to induce potent HIV-1-specific immunity. However, despite these findings, several candidates have demonstrated limited efficacy in viral replication control. In this study, we used the PubMed and ClinicalTrial.gov databases to review the rationale of designing curative HIV-1 vaccine immunogens based on the conserved favorable site of the virus. Most of these studies evaluate the efficacy of vaccine candidates in combination with other therapeutics and/or with new formulations and immunization protocols. This review briefly describes the design of conserved multiepitope constructs and outlines the results of these vaccine candidates in the recent clinical pipeline.
The Human Immunodeficiency Virus-1 (HIV-1) remains a global public health, particularly in low-income developing countries.1 Based on the UNAIDS estimates, HIV-1 has affected 84.2 million people since the start of the epidemic with around 1.5 million new infections in 2021. It was approximated that 38.4 million people globally were living with HIV-1 in 2021 and 28.7 million people were Receiving Antiretroviral Therapy (ART) (https://www.unaids.org/en/resources/fact-sheet). Highly Active Antiretroviral Therapy (HAART) has proven as a standard treatment for effective prevention of HIV-1-associated clinical progression.2 HAART successfully reduces virus replication in targe T-cells and HIV-1 transmission risk, but it cannot eradicate the infection and suppress the Plasma Viral Load (pVL).3 Moreover, acute and chronic drug toxicities, development of drug-resistant strains, and high cost of lifelong antiretroviral therapy are the most important problems of antiretroviral drugs.4,5 Therefore, the development of an inexpensive prophylactic and/or therapeutic HIV-1 vaccine has been proposed as an intense need. Prophylactic vaccines are the most promising solution to stop the HIV-1 pandemic, but they have shown repeated failures in phases II and III human clinical trials.6,7 Up to now, the most effective prophylactic vaccine is RV144 (ALVACHIV-1 (vCP1521) viral vector prime/AIDSVAX B/E gp120 protein boost) achieved to phase III clinical trial with 31% efficacy and limited durability.8 Biological obstacles to HIV-1 vaccine development originate from virus characteristics such as high mutation and recombination rate during viral replication, genetic variability and cell-associated spreading of the virus.9,10 Also, the lack of an appropriate animal model for AIDS, and limited information about the immunological correlates of HIV-1 protection are the scientific challenges toward vaccine achievement.11,12 There is no approved prophylactic HIV-1 vaccine for clinical use, and the number of new HIV-1-infected people continues to expand. Thus, development of an effective therapeutic vaccine as a strategy to tackle HIV-1 persistence and cure infected patients would be a valuable advance.13 Furthermore, the lack of a definitive cure for HIV-1 infection enhances the importance of therapeutic vaccines as an alternative to the HAART.14 So far, due to HIV-1 genetic diversity and its escape from the immune system, different therapeutic vaccines have not been successful in eradicating the virus. Therefore, novel therapeutic vaccine candidates are developing as curative strategies for HIV-1 infection. New studies often evaluate the efficacy of vaccine candidates (e.g., conserved multiepitope vaccine constructs) in combination with other therapeutics and/or with new formulations and immunization protocols. This review briefly describes the design of these conserved multiepitope constructs and outlines the results of these vaccine candidates in the recent clinical pipeline.
HIV-1 genome integration and latencyHIV-1 genome was composed of a single-stranded RNA (ssRNA) which encodes the structural (Gag and Env), functional (Pol), accessory (Nef, Vif, Vpu & Vpr), and regulatory (Tat and Rev) proteins. Moreover, long-terminal repeats (LTRs) were located at two ends of this ssRNA.15 During the HIV-1 infection, the Gag, Pol, Env and Nef proteins are mainly expressed and also targeted by host immune system.16 The LTRs are also crucial for the integration of ssRNA into the host cell genome as a proviral genome by reverse transcription.17 Proviruses don't have active viral replication and antigen expression especially in memory CD4+ T-cell populations. Formation of these latently infected cell reservoirs is started early during the HIV-1 infection in diverse cell types and multiple anatomical sites with limited drug penetration.18 Thus, HIV-1 infection turns into an incurable disease because the integrated replication-competent proviruses become invisible to the immune system and not susceptible to ART.18 Furthermore, virus production, disease progression and virus spreading can rapidly develop after cessation of ART.19 Thus, it is not surprising that these viral reservoirs are one of the main obstacles to achieve a definitive HIV-1 cure.
Correlation of immune responses and HIV-1 controlThe obtained evidences from HIV-1 elite controllers (Pvl < 50 copies/mL) and viraemic controllers (pVL: 50‒2000 copies/mL) demonstrated that virus-specific cellular immune responses are responsible for viral replication control.9,20 This effective T-cell immunity targets particularly the conserved HIV-1 regions (i.e., the limited mutationally regions). Effective cytotoxic CD8+ T-Lymphocytes (CTL) recognize viral peptides on the HLA class I peptide-binding groove in infected cells.3,9 Furthermore, Helper CD4+ T-Lymphocytes (HTL) facilitate the induction of optimal CTL responses.21 Therefore, recruiting improved T-cell-directed strategies is critical in therapeutic vaccine settings. Unlike cellular immunity, there is little evidence for strong correlations between humoral immunity and spontaneous HIV control.22 Neutralizing antibodies could be detected in 20%‒30% of individuals living with HIV-1 infection. These antibodies only target the Envelope glycoprotein (Env) that is responsible for viral entry.20,23 Antibody-Dependent Cellular Cytotoxicity (ADCC) or Phagocytosis (ADCP) are the effector functions of antibodies to hasten the clearance of already infected cells, and control the progression of disease.1 Some clinical studies demonstrated that viremia suppression, and delayed virus rebound could be achieved by passive infusion of broadly neutralizing antibodies.24,25 These antibodies are directed to conserved HIV-1 epitopes, to overcome viral variability.22 Thus, it is hypothesized that both cellular and humoral immunity can be recruited by conserved multiepitope HIV-1 therapeutic vaccines.14,15 The therapeutic vaccination platform expands and re-educates virus-specific immune responses (particularly CD8+ T-cell response) to kill infected cells before production of progeny virions.26 This platform limits the replenishment of viral reservoirs to achieve a functional cure in the absence of ART.
Rational HIV-1 therapeutic immunogen designHIV-1 has high viral diversity, and the host immune system in individuals living with HIV-1 infection focuses on immuno-dominant sites of the virus genome that are variable.27 HIV-1 CTL escape pathway enhances viral fitness and pathogenesis, and renders the infected cells insensitive to cell cytotoxicity.16 Thus, the CTLs specific to an immuno-dominant HIV-1 epitope cannot recognize an infected CD4+ cell due to the escape mutation.28 In contrast, HIV-1 elite controllers and long-term non-progressors have effective CTL responses specific for those subdominant epitopes that are not frequently targeted in natural infection. These epitopes are structurally conserved and have fewer mutations in HLA anchor and T-Cell Receptor (TCR) contact sites.9 Several lines of evidences suggest that redirecting host immunity toward specific invariant sites of HIV-1 proteome enhances the specificity and breadth of the immune response and constrains viral immune escape.29 Conventional vaccines harboring the full-length immunogens induce immunity against immuno-dominant sites that are evaded from immune recognition by mutational escape.14 In contrast, novel immunogens containing multiple mutationally intolerant sites may protect host immunity from viral escape and viral rebound following ART interruption.30 Novel immunogens based on the conserved approaches can be constructed by stretches of large and relatively conserved protein regions (conserved-region vaccine approach) or short peptides harboring highly conserved or functional epitopes (conserved-element approach).27 Recruiting HIV-1-derived site with greater conservation, offers the optimal match of consensus sequences between various viral strains.25,31 Since every MHC class I molecule presents a restricted set of HIV-1-derived 8–11-amino acid peptides, thus antigenic specificity of CD8+ T-cells depends on the binding properties of HLA class I alleles (Fig. 1).14 Thus, targeting the maximum number of epitopes in a novel immunogen may be the most efficient pathway to increase HLA types coverage.32 Multiepitope immunogens can also enhance magnitude and breadth of T-cell responses to cover HIV-1 diversity.33 Although sequence conservation is a sensible solution for immune evasion constraint, it may be insufficient as only some subsets of conserved sites are mutational intolerant.26 Thus, inclusion of the protective epitopes associated with lower pVL in a conserved multiepitope immunogen can boost its efficacy, as well.30 Progress in computational vaccinology approach and in-silico epitope-mapping has been instrumental in identification of both conserved and protective epitopes, and rational design of novel immunogens.25,26,31 Conserved multiepitope immunogens are also expected to avoid the induction of responses to potential decoy targets capable of inducing adverse immune reactions.34 Therefore, conserved multiepitope approach is an attractive modality for HIV-1 therapeutic vaccine which can stimulate long-lasting protection, and achieve sustained functional cure.31,35,36
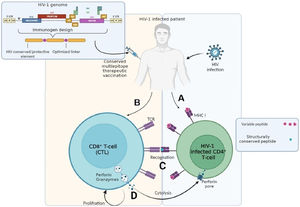
Induction of cell-mediated immunity by a conserved multiepitope HIV-1 therapeutic vaccine: (A) Productively HIV-1 infected CD4+T-cell in an HIV-1 infected paitent displays both variable and conserved epitopes of viral peptides via MHC-I molecules; (B) Designed HIV-1 therapeutic vaccine based on the conserved sites of HIV-1 genome can activate Cytotoxic T Lymphocytes (CTLs) in the HIV-1 infected individuals; (C) Effector-specific CTLs in a vaccinated patient recognizes the conserved viral peptides better than variable ones by T-cell receptors (TCRs); (D) Recognition of the conserved epitopes induces proliferation and perforin/granzyme secretion in poly-functional CTLs.
Because of tremendous HIV-1 genetic diversity, the T-cell-based therapeutic vaccine faces the same challenge as an antibody-inducing preventive vaccine.22 A large number of HIV-1 therapeutic vaccine candidates have been tested in clinical trials, and none of them could able to completely prevent virus rebound following Analytical Treatment Interruption (ATI). Moreover, several candidates are currently under evaluation. To achieve an optimal effect of therapeutic vaccination, designing the immunogens and choice the vectors are the critical steps.11,31 Innovative immunogens based on the conserved multiepitope approaches are theoretically promising to optimize coverage, specificity and immune stimulation of vaccines, and reduce viral immune evasion.15,30 Herein, we reviewed the last 15 years of clinical studies toward an HIV-1 therapeutic vaccine based on the conserved multiepitope approaches. The design of immunogens was directed toward inducing HIV-1-specific responses. The immunogen candidates in different vaccine platforms based on nucleic acids (DNA, mRNA), viral vectors, Dendritic Cells (DCs), peptides or proteins were reviewed and described as following. The clinical results of each vaccination strategy based on conserved multiepitope immunogens were listed in Table 1.
Clinical trials of therapeutic conserved multiepitope vaccine immunogens in different vaccine platforms.
Ab, Antibody; ART, Antiretroviral Therapy; ATI, Analytical Treatment Interruption; CAF01, Cationic Adjuvant Formulation number 1; ChAdV63, Chimpanzee Adenovirus serotype 63; CTL, Cytotoxic T-Lymphocyte; HAART, Highly Active Antiretroviral Therapy; HLA, Human Leukocyte Antigen; HTL, Helper T-lymphocyte; MPLA, Mono-Phosphoryl Llipid A; MVA, Modified Vaccinia Virus Ankara; PTE, Potential T-cell Epitope; pvDNA, Plasma Viral DNA; pVL, Plasma Viral Load.
Hanke and colleagues have presented the first HIV-1 conserved-region vaccine as a chimeric immunogen. This immunogen named as HIV-1consv, was constructed based on the consensus sequence of four HIV-1 proteins.37 The HIV-1consv contained 14 highly conserved cross-clade regions (between 27 and 128 amino acids [aa], a total of 778 aa) presented by distinct HLAs.37 Preclinical studies demonstrated the high immunogenicity of HIV-1consv with different vaccine modalities in mice and macaques.37,38 It was also administered in healthy adults as preventive strategy in different clinical trials, and showed safety and immunogenicity profiles.39 Moreover, induction of CD8+T-cells response with strong HIV-1 inhibition capacity in vitro was reported in all vaccine recipients.40-42 In a phase I clinical trial, HIV-1Consv was delivered by Modified Vaccinia Virus Ankara (MVA) in a homologous prime/boost regimen to virologically-suppressed individuals living with HIV-1 infection (ClinicalTrials.gov Identifier: NCT01024842). The therapeutic MVA/HIV-1consv vaccine showed modest immunogenicity in vaccinated individuals.43 It was also administered intramuscularly to newly HIV-1 infected patients in a heterologous prime/boost regimen (ClinicalTrials.gov Identifier: NCT01712425 (BCN01)). In this phase I clinical trial, the HIV-1consv gene was inserted into an attenuated chimpanzee adenovirus serotype 63 (ChAdV63) vector as prime and the MVA vector as boost.44 The ChAd-MVA/HIV-1consv vaccination was safe and immunogenic. It was also able to re-direct pre-existing HIV-1-specific T-cell responses to vaccine-encoded conserved segments in all participants. Additionally, the ChAd-MVA/HIV-1consv vaccine had high viral inhibition capacity in vitro, but showed no effect on the viral reservoirs.39,43 On the other hand, 15 participants who had shown sustained viral suppression in BCN01 trial were given two booster doses of MVA.HIV-1consv combined with Romidepsin (RMD) in BCN02 study (ClinicalTrials.gov Identifier: NCT02616874). BCN02 was a proof-of-concept clinical trial that combined therapeutic vaccination and Latency-Reversing Agent (LRA) to reactivate the latent viral reservoirs and make them sensitive to vaccine-induced immune clearance.17,45 RMD is a histone deacetylase inhibitor (HDACi) that acts as a latency-reversing agent.39,46 It had the highest activity on histone acetylation and HIV-1 replication in several studies.47 BCN02 vaccination induced significant reduction of the viral reservoirs and refocused broad HIV-1consv-specific T-cells responses toward the conserved regions of HIV-1. After a 12-week ATI, 23% of recipients showed durable suppression of viremia for up to 32 weeks without viral reservoir reseeding.46 In the RIVER study (Research in Viral Eradication of HIV-1 Reservoir), heterologous prime/boost regimen of ChAdV63.HIV-1consv-MVA.HIV-1consv vaccine plus a 28-day course of Vorinostat (HDACi) was administered in newly HIV-1 infected individuals (ClinicalTrials.gov Identifier: NCT02336074).48 Histone acetylation induced by vorinostat and HIV-1-specific T-cell responses were achieved in vaccinated individuals. However, there was no significant pro-viral reduction in the vaccinated group compared to group receiving ART alone.48-50
tHIV-1consvXtHIV-1consvX was the second generation of HIV-1consv immunogen that was computationally designed and improved. The tHIV-1consvX utilized bivalent complementary mosaic proteins containing 6 conserved regions of Gag and Pol. The tHIV-1consvX regions were more conserved than 14 regions in the HIV-1consv and could minimize junctional epitopes.39 The tHIV-1consvX encoded 895 amino acids and had the maximum number of Potential T-cell Epitopes (PTEs). PTEs were associated with low pVL and had perfect match to 80% of M group strains.51-53 This immunogen had the maximized global epitope matching and could accommodate human HLA class I diversity by the maximal number of PTEs and amino acids.54 In preclinical evaluation, the self-amplifying tHIV-1consvX mRNA had strong immunogenicity, and could elicit T-cells and memory responses in mice.55 Furthermore, lower viral loads and higher CD4+ T-cell counts in chronic individuals living with HIV-1 infection (untreated) were observed after immunization with tHIV-1consvX vectored by a combination of DNA, simian adenovirus and MVA.56 MVA-vectored therapeutic vaccines expressing tHIVconsv3 and tHIVconsv4 immunogens were recently evaluated in a double-blind, randomized phase I clinical trial in untreated chronic individuals with durable viral suppression (HIV-1 RNA <50 copies/mL) (ClinicalTrials.gov Identifier: NCT03844386). It was generally safe with no serious adverse reaction in participants.
LIPO-5LIPO-5 immunogen was composed of five HIV-1 peptides (Nef 66–97, Nef 116–145, Gag 17–35, Gag 253–284 and Pol 325–355) coupled to a palmitoyl tail. These peptides contained multiple conserved CD8+and CD4+T-cell epitopes.57 The LIPO-5 vaccine was firstly tested in phase I and II placebo-controlled ANRS trials in 28 HIV-1-uninfected volunteers and elicited sustained specific T-cell responses in >85% of the vaccinees.58,59 The DC-based therapeutic vaccine (DALIA) was an ex vivo-generated IFNα Dendritic Cells (DCs) loaded with LIPO-5, and completed phase I clinical trial (clinicaltrials.gov identifier: NCT00796770).60 DALIA vaccination was safe and also enhanced HIV-1-specific immune responses in the vaccinated group. It could elicit and/or expand HIV-1-specific CD8+T-cells producing IFN-γ, perforin, granzyme A and granzyme B.61,62 Virus rebound was observed after 14 days of ATI in volunteers. The CD4+T-cells secreting IFN-γ, IL-2 and IL-13 were also detected in the vaccinees with lower viral replication peaks following ATI. The intramuscular administration of GTU-MultiHIV-1 (DNA vaccine encoding a fusion protein of Rev, Nef, Tat, p17 and p24) prime and LIPO-5 boost regimens in chronic asymptomatic individuals living with HIV-1 infection was carried out in a phase II trial (clinicaltrials.gov identifier: NCT01492985).62 This vaccination setting was safe and immunogenic but had no impact on viral replication control after a 12-week ATI.
AFO-18AFO-18 was a synthetic immunogen that contained 15 peptides representing subdominant conserved CTL epitopes and 2 peptides representing HTL epitopes. These peptides were derived from six HIV-1 proteins of different subtypes in addition to one universal HTL PADRE-peptide.63 The CTL epitopes binding to the HLA-supertype were predicted by artificial neural networks and theoretically covered more than 90%‒100% of different populations.64 The peptides were mixed with a Cationic Adjuvant Formulation number 1 (CAF01), and could induce T-cell responses in mice.63 CAF01 is a synthetic liposomal adjuvant capable of inducing CD4+ T-cell immunity and activating functional CD8+ T-cells using minimal HIV-1 peptides.65 Immunization of ART-naive individuals living with HIV-1 infection by AFO-18 peptides mixed with CAF01 adjuvant was carried out in two phase I clinical trials in Denmark and West Africa (ClinicalTrials.gov Identifier: NCT01009762 and NCT01141205). The vaccination was safe, and could induce new vaccine-specific T-cell responses in recipients.66,67
HIV-1-vHIV-1-v was an admixture of four polypeptides containing in silico-identified short, conserved domains of four HIV-1 proteins. These multiepitope domains encode 20 to 50 amino acids in the consensus sequences with ≥70% conservancy for the HIV-1 strains and high affinity to HLA alleles.68 The HIV-1-v was immunogenic in mice, and in untreated individuals living with HIV-1 infection in phase Ib clinical trial (ClinicalTrials.gov Identifier: NCT01071031). Single-dose vaccination by HIV-1-v adjuvanted with ISA-51 was safe and could elicit T- and B-cell responses in 75% and 45% of participants, respectively. Viral load reduction with no change in CD4+counts was also observed.69
HTIMothe et al. designed HTI (HIV-1ACAT T cell Immunogen) based on HIV-1 regions associated with relative viral control. For selection of the viral targets, protective T-cell responses from more than 950 chronic ART-naive patients were evaluated toward Overlapping Peptides (OLPs) spanning the entire HIV-1 proteome. 26 OLPs targeted by participants with low viral loads were identified in five HIV-1 proteins.52 These selected regions (between 11 and 78 aa) were conserved and cross-reactive to different HIV-1 variants and provided the basis of the HTI construction. HTI was composed of 16 regions containing more than 50 optimal CD4+ and CD8+ T-cell epitopes that were restricted by broad HLA-I and HLA-II alleles.70 For optimal processing, the selected fragments were joined by polyalanine-enriched linkers, and the final polypeptide sequence encoded 529 amino acids.70 Immunization of rhesus macaques and mice using different modalities of HTI combined with TriMiX could induce broad and balanced T-cell responses.70,71 TriMiX composed of a mixture of activating molecules (CD40L + CD70 + constitutively active variant of Toll Like Receptor 4 (caTLRA4)) that was functional in the activity of APCs (especially DCs), and could co-activate specific T-cells. Three doses of the naked mRNA encoding HTI in combination with TriMiX mRNAs were delivered intranodally to chronic individuals living with HIV-1 infection in phase I and IIa clinical trials (ClinicalTrials.gov Identifier: NCT02413645 and NCT02888756). This vaccine was safe with moderate immunogenicity and T-cell response induction, but it had no efficacy in controlling viral rebound.29,72
The safety, immunogenicity, and/or efficacy of HTI with different formulations have been evaluated in several human clinical trials. The AELIX-002 was a randomized, double-blind phase I/II clinical trial started in 2017 in newly HIV-1 infected patients. In AELIX-002, HTI antigen was administered in a heterologous prime/boost regimen (DNA.HTI (D) prime/MVA.HTI (M) and ChAdOx1.HTI (C) viral vector boost) (ClinicalTrials.gov Identifier: NCT03204617). Vaccination was safe and highly immunogenic in 97% of recipients who completed the DDDMM followed by CCM vaccination. Anti-HIV-1 T-cell response was HTI-specific with a prolonged time off ART in 40% of vaccinees.73 In the other study, 6 AELIX-002 participants with pVL less than 2000 copies/mL after 24 weeks of ATI has undergone a one-year extension of the ATI phase (total duration of ATI was 72 weeks) (ClinicalTrials.gov Identifier: NCT04385875).13,74 This study evaluated the safety and durability of viral control beyond 24 weeks of ATI. There are no posted results in clinicaltrials.gov for this study.
The AELIX-002 encouraging data supported the use of HTI in the second clinical trial named as AELIX-003. It is a Phase IIa randomized, double-blind trial of MVA.HTI and ChAdOx1.HTI vaccine combined with the TLR7 agonist Vesatolimod (GS-9620) (ClinicalTrials.gov Identifier: NCT04364035). After vaccination and TLR-7 agonist treatment, the participants will proceed to an ATI phase. Safety and efficacy of the vaccine, and also viral replication control ability in the absence of anti-retroviral drugs will be assessed in vaccinees. Data from this trial is expected in late 2022.
In the other Phase I/IIa clinical study, co-administration of HTI with a single bNAb targeting the Env V3 loop (10–1074) and the LRA RMD was evaluated (ClinicalTrials.gov Identifier: NCT03619278).17 The 10–1074 Ab is a potent anti-HIV-1 neutralizing antibody that binds to the V3 glycan on the gp120.75 It is hypothesized that LRA can induce Env expression on the surface of latently infected cells, and make them an accessible target for broadly neutralizing antibodies.76 Thus, the safety, immunogenicity and effectiveness of the combined HTI+Ab+LRA vaccination were evaluated in participants who were randomized into 4 study arms. Participants were immunized with different doses of mRNA HTI + TriMix, MVA.HTI, 10‒1074 Ab and RMD according to their study arms. There are no posted results in clinicaltrials.gov for this trial.
In recent years, progress has been made to develop native-like Env trimers as a target for broadly neutralizing antibodies. The first in-human clinical trial of native-like Env trimers was carried out in 2018.77 The safety and immunogenicity of a recombinant Env protein mimicking the native trimer in addition to the ChAdOx1.HTI and MVA.HTI adjuvanted with MPLA (mono-phosphoryl lipid A) liposomes is currently being tested in a phase I randomized, double-blind study named as BCN03 (ClinicalTrials.gov Identifier: NCT05208125). The recombinant Env in BCN03 study, displays group M consensus B-cell epitopes (ConM SOSIP.v7 gp140). Moreover, the MPLA is a potent adjuvant for inducing antibodies against the Env by mimicking the lipid bilayer of virus surface.78 Intramuscular administration of ChAdOx1.HTI at week 0, ConM SOSIP.v7 at weeks 4, 12 and 28, and MVA.HTI at week 22 was carried out in virologically suppressed ART-treated HIV-1 positive individuals. The results will be assessed in near Future. Indeed, at week 30, safety and immunogenicity will be measured, and all participants will undergo a 24-week ATI. ART will be resumed at week 54 and participants will be followed during an additional safety period of 12 weeks. Data from this trial is expected in May 2023.
Gag and pol conserved peptidesGag and Pol conserved peptides were designed based on the ultra-conserved elements (minimum length is 14 amino acids and included at least 5 PTEs). These peptides were used for the construction of a DC-based therapeutic vaccine. The autologous dendritic cells were matured with an optimized cocktail (a1DC) or with a standard prostaglandin E2 cocktail (pgDC). Then, the mature DCs were loaded with Gag and Pol conserved peptides pool or autologous inactivated whole HIV-1 virus. These vaccines were tested in a randomized phase I clinical trial (ClinicalTrials.gov Identifier: NCT03758625) to evaluate the safety, tolerability, and immunogenicity. Data from this trial is expected in June 2023.
Multiepitope immunogens based on a single HIV-1 antigenIn addition to the mentioned multiepitope vaccine trials, several therapeutic HIV-1 vaccines based on the highly conserved regions from one single HIV-1 protein have been evaluated in different clinical trials. As shown in Table 1, the NCT00659789, NCT01712256, NCT01473810, NCT01704781 and NCT02092116 clinical studies have assessed the Vacc-4x efficacy. Furthermore, the NCT03560258 and NCT04357821 are two recent clinical studies that are testing the effectiveness of Gag Conserved Elements (p24CE) in therapeutic vaccination setting.
Vacc-4xVacc-4x was prepared from four peptides containing conserved regions from HIV-1- Gag p24. This vaccination has efficacy in induction of proliferative T-cell responses. Intradermal and intranasal administrations of Vacc-4x with the recombinant human granulocyte-macrophage colony stimulating factor (rhuGM-CSF) named as Leukine and Endocin adjuvants led to significant difference in pVL between placebo and vaccinated groups in phase I and II clinical trials.79-82 Vaccination by Vacc-4x in combination with Lenalidomide (an immune-modulator) significantly increased mean CD4+ T-cell counts in patients with low pre-ART CD4+ counts.1 Furthermore, vaccination by Vacc-4x combined with rhuGM-CSF and Romidepsin induced a reduction in the latent HIV-1 reservoirs in the phase Ib/IIa trial.83,84
HIV-1-Gag p24HIV-1-Gag p24 was constructed by two plasmids encoding seven highly conserved elements of HIV-1-Gag p24, associated with viremia control and restricted by broad HLAs. In a phase I/II clinical trial, a DNA vaccine named as p24CE1/2 pDNA was electroporated alone or mixed with the full-length 55^gag pDNA vaccine to individuals living with HIV-1 infection. It was generally safe with no serious adverse reaction in participants. The p24CE vaccine is currently being administrated in a combination therapy with IL-12, MVA/HIV-162B encoding Gag, Pol and Env, broadly neutralizing antibodies (CD4 binding VRC07–523LS Abs and 10–1074 Abs), and LRA Lefitolimod (a TLR9 agonist) in a Phase I/II clinical study. Data from this trial is expected in December 2024.
Immunogens based on a single domain of HIV-1 antigensSome therapeutic HIV-1 vaccine candidates were synthesized based on the isolated HIV-1 protein domain and evaluated in different clinical trials as briefly shown in Table 2. The NCT00848211, NCT01144026 and NCT01335191 have evaluated the TUTI-16, the NCT01627678 have tested the Vacc-C5, and the NCT01549119, NCT02041247 and NCT02390466 have investigated the efficacy of VAC-3S immunogen in therapeutic setting.
Clinical trials of therapeutic vaccine immunogens based on the isolated HIV-1 domain.
Ab, Antibody; ATI, Analytical Treatment Interruption; ART, Antiretroviral Therapy.
TUTI-16 was a synthetic self-adjuvating lipopeptide based on a universal anti-Tat epitope. This epitope induces antibodies that are reactive with all eight variants of Tat. This vaccine was assessed in both preventive and therapeutic strategies. In therapeutic setting, the TUTI-16 was safe and could induce high levels of anti-Tat Abs that block the circulating Tat function. Highly significant reduction of HIV-1 pVL was obtained in the lowest vaccine dose. However, it was ineffectual in controlling HIV rebound after ATI.85-87
Vacc-C5Vacc-C5 was synthesized as a complex peptide based on the 5th Constant (C5) domain on the HIV-ENV-gp120. It consisted of 2 peptide domains (i.e., the gp120-C5 domain, and the highly conserved gp41-KE domain). High titers of antibodies to the C5 domain were inversely related to HIV-1 disease progression. The Vacc-C5 therapeutic vaccine was safe and could induce mild humoral and cellular immune responses when administered intramuscularly with Alhydrogel or intradermally with GM-CSF as an adjuvant. Vacc-C5-specific CTL proliferative responses were also increased after the first booster period.33,88
VAC-3SVAC-3S was a peptide-based therapeutic vaccine comprised of a highly conserved Env gp41 motif (3S multiepitope peptide). It was evaluated with or without the CRM197 carrier protein in phase II and I/IIa clinical trials. It was safe and could induce high levels of anti-3S Abs correlated with an increase in CD4+ T-cell survival, CD4+/CD8+ ratio, and total HIV-1 blood reservoirs reduction.2
Vaccine nanoparticles for HIV-1Nanotechnology represents a potential solution for development of immunogens in HIV-1 vaccines.89 Recently, the Nanoparticles (NPs) have attracted a special interest for development of HIV-1 subunit vaccines. New NP platforms are now used to improve immunization strategies against HIV-1 infection.90 The use of nanoparticles for prophylactic HIV-1 vaccination has several advantages including safety profile provided by biocompatible biomaterials, in vivo stability of immunogens, improved targeting to APCs, increased phagocytosis and processing of antigens, and multivalent presentation of antigens for induction of sustained IgG production.91 Indeed, nanovaccines are able to deliver and present antigens in native-like conformation, and increase immunogenicity against recombinant antigens alone. Up to now, various types of nanovaccines have been improved such as Viral Like Particles (VLPs), naturally occurring or rationally designed protein assemblies, liposomes, and lipid nanoparticles. These nanovaccines can be engineered to present antigens on their surface to increase immunogenicity and/ or deliver soluble antigens or immunostimulatory molecules in their cores to enhance the efficiency of vaccine.89 However, further researches are required to use nanoparticles in the development of an effective and safe HIV-1 multiepitope vaccine in clinical trials.
DiscussionBased on the limitations of HAART and the failure of therapeutic vaccine candidates in HIV-1 cure, new approaches should be developed to eradicate the virus or make durable remission of HIV-1 infection.26 An ideal HIV-1 therapeutics should have potency in the induction of immune responses against all HIV-1 variants. Recruiting multiple conserved epitopes in a chimeric immunogen may be a promising strategy to induce these cross-reactive immune responses. It is hypothesized that functional CD8+ T-cells in HIV-1 controllers lacking protective HLA alleles preferentially target ‘networked’ regions of HIV-1 proteins.20,92 The networked regions are structurally conserved/constrained sites in the HIV-1 proteome.20 Mutation in the networked residues leads to intense viral fitness reduction.9,20 These regions have been used in different therapeutic immunogens (mostly containing the Gag and Pol proteins) in human clinical trials that were generally immunogenic, safe and specific. These HIV-1 therapeutic vaccines can be used as alternatives to ART or as additional cure to intensify ART effect, not to replace it.92 Conjunction of therapeutic vaccines with ART increases the effectiveness of ART, and leads to viral replication control.19 Moreover, individuals who initiate ART early after HIV-1 acquisition (in the first six months) would be more likely to response to vaccination successfully.19,47 They have more preserved functional immunity (e.g., individuals in NCT03204617 and NCT04364035 trials). In patients not receiving ART, the viral reservoir becomes almost completely dominated by CTL-resistant variants.92 As shown in Tables 1 and 2, just 5 conserved multiepitope therapeutic vaccines (i.e., NCT01141205, NCT01009762, NCT01071031, NCT00848211 and NCT01144026) were tested in the untreated individuals with ART.
However, hiding the latent viruses in viral reservoirs is a main hurdle in HIV-1 eradication. Thus, these cells can be mobilized by pharmacological interventions16 (Fig. 2). Reactivation of latently infected cells by LRAs and removing these cells by therapeutic T-cell vaccines (shock and kill strategy) have been assessed in several clinical trials with different classes of LRAs.28,93 Of these LRAs, the Histone Deacetylase Inhibitors (HDACi) were widely used in clinical trials.94 Vorinostat and Romidepsin are the HDACi that were used in four conserved multiepitope therapeutic vaccine trials as shown in Table 3 (i.e., NCT02616874, NCT03619278 and NCT02336074, NCT01704781). Histone deacetylation mechanism compacts the chromatin and hinders gene expression in latent cells. Inhibiting histone deacetylation by vorinostat or Romidepsin increased production of HIV-1 RNAs in the viral reservoirs, and improved CTLs recognition.95 Some Toll-Like Receptor (TLR) agonists are the other classes of anti-latency drugs.96 The Vesatolimod and the Lefitolimod are TLR7 and TLR9 agonists which can activate T- and NK- cells, and reduce the viral reservoirs.24 They have been used in two ongoing multiepitope vaccines clinical trials (i.e., NCT04357821 and NCT04364035).
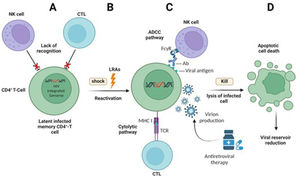
The effects of Latency-Reversing Agents (LRAs) on the HIV-1 reservoir reduction by “shock and kill” strategy: (A) Latently infected CD4+ T-cells containing the integrated HIV-1 genome are not recognized by Cytotoxic T-lymphocytes (CTLs) and natural killer (NK) cells; (B) LRAs can reactivate gene transcription in the HIV-1 latent cells (shock); (C) The transcriptionally active HIV-1 infected cells can be recognized by CTLs and NK cells. Then, immune-mediated killing of the infected cells is carried out by Antibody-Dependent Cellular Cytotoxicity (ADCC) and cytolytic pathways. Newly produced virions are also deleted by ART drugs (kill); (D) Apoptotic cell death of infected cells makes a reduction in the size of HIV-1 latent reservoir.
Summary of conserved multiepitope vaccines in combination with other therapeutics.
ATI, Analytical Treatment Interruption; ART, Antiretroviral Therapy; CAF01, Cationic Adjuvant Formulation number 1; DC, Dendritic Cell; HDACi, Histone Deacetylase Inhibitor; IL, Interleukin; LRA, Latency-Reversing Agent; NAb, Neutralizing Antibody; rhuGM-CSF, Recombinant Human Granulocyte-Macrophage Colony Stimulating Factor; TLR, Toll Like Receptor; TriMix: CD40L + CD70 + Constitutively active variant of TLR4 (caTLRA4).
Signs: + shows the used therapeutics along with vaccines; - shows the lack of therapeutics in vaccine constructs.
HIV-1 therapeutic vaccines or other immune-based therapies showed a limited efficacy when tested alone. Thus, it has been proposed that different interventions should be co-administered and evaluated in novel combinational approaches. Table 3 shows the clinical trials of conserved multiepitope immunogens combined with other therapeutics. Several encouraging data demonstrated that using broadly neutralizing antibodies can induce variable levels of HIV-1 control by mediating ADCC and blocking viral replication (Fig. 2).93 Recently, Borducchi et al. suggested that using broadly neutralizing antibodies combined with LRAs and other immune modulators can harness the full capacities of antibodies during ART cessation.47,97 In this method, activation of gene expression by LRAs in the latent viral reservoirs led to an improved virus neutralization in the presence of broadly neutralizing antibodies.98 This strategy was very promising in Non-Human Primates (NHPs), and has been evaluated in two multiepitope vaccines clinical trials (i.e., NCT03619278 and NCT04357821). Using immuno-modulators and cytokines such as IL-12 and IL-15 are the other interventions that enhance the efficacy of conserved multiepitope vaccines. IL-12 strongly stimulates the NK cells and CTL maturation and has been used in an ongoing combinational trial on p24CE1/2 vaccine as well as broadly neutralizing antibodies and TLR agonist (NCT04357821). Besides, several data showed that using IL-15 in a combined therapeutic vaccine setting could enhance the number of active CTLs in sanctuary sites such as B-cell follicles in lymph nodes.19 Sanctuary sites are the tissues with poor CTL and ART penetration. These sites are favorable locations for the viral reservoir formation. Penetration of CTL into these tissues at sufficient numbers is an additional challenge for HIV treatments. Additional strategy for removing the infected cells from these sites is the use of PD-1-directed immunotherapy (immune checkpoint blockade) to reverse immune dysfunction and HIV-1 latency. Developing CD8+ T-cells expressing follicle-homing receptor CXCR5+is the other proposed pathway for this purpose.19,20
After administration of combinational therapeutic vaccines, measurement of the HIV-1 reservoir size and assessment of viral rebound followed ATI are actual clinical endpoints.99 Plasma HIV-1 RNA measurement is the only FDA-approved clinical efficacy measure for evaluation of viral rebound after ATI. Although ATI is not without risks and has ethical and public health concerns, it remains a main component in clinical trial efficacy studies.89,99 Among all the mentioned trials, 14 of them had no ATI period. In addition, further assays need to be developed and validated so that they can be used for monitoring the impact of a potential therapy on clinical parameters such as the HIV reservoirs, viral replication and CD4+ count.
Furthermore, clinical, and experimental evidence showed that the Tat protein has a critical role in maintenance and replenishment of the HIV-1 reservoirs. Therapeutic vaccines based on the Tat protein are one of the most advanced therapeutic vaccine approaches for ART intensification. Clinical data of these vaccines showed that combination therapy of Tat immunogens with ART drugs could increase the number and function of CD4+ and CD8+ T-cells, and reduce pro-viral load.1
ConclusionsIn conclusion, development of an effective HIV-1 therapeutic vaccine needs the rational design of immunogens to redirect responses to multiple known protective and un-mutated parts of the virus that cover distinct HLAs and HIV-1 diversity. Quality of vaccine-induced immunity including poly-functionality, proliferation and cytotoxicity is the most important issue of therapeutic vaccines. Although data from clinical trials demonstrated that the quality of immune response can be optimized by a conserved multiepitope vaccine, there are several potential challenges for increasing the longevity and durability of these immune responses. Maintaining adequate numbers of poly functional and specific CTLs within tissues and peripheral blood, recovering immune exhaustion, and reversing viral latency are the other concerns. While immunogen and adjuvant design, and selection of appropriate vector have been widely investigated, combining therapeutic vaccines with complementary interventions is underdeveloped. The latency-reversing and immunomodulatory agents, broadly neutralizing antibodies or passive immune therapy, cytokines, and checkpoint inhibitors are different strategies to co-administer with conserved multiepitope vaccines. Using combined approaches appears to be one of the best options to succeed in achieving a functional HIV-1 cure.