Visceral leishmaniasis is a relevant public health problem worldwide. Most of the reported cases in Latin America are from Brazil. Herein we report two human cases of congenitally transmitted visceral leishmaniasis in two patients who developed symptoms during pregnancy. The diagnosis was made by visual examination of Leishmania parasites in bone marrow aspirates of the mothers and by detecting parasite kDNA in bone marrow samples of the newborn children using polymerase chain reaction.
American visceral leishmaniasis (AVL) is an endemic disease caused by Leishmania infantum (syn. L. chagasi), which occurs predominantly in tropical and subtropical regions of South America. AVL is typically transmitted to humans through the bite of the female sand fly Lutzomyia longipalpis. Other routes of transmission have been reported in humans such as sharing of contaminated needles among drug users,1 organ transplants2 and congenital transmission.3 Classic manifestations of AVL are persistent fever, weight loss, progressive anemia or pancytopenia, hypergammaglobulinemia, hepatomegaly and splenomegaly. Congenital transmission of visceral leishmaniasis was first reported by Low and Cooke4 in Africa and by 2011, nine cases of AVL were reported in pregnant women in Brazil.5–8 Congenitally transmitted visceral leishmaniasis has a course of disease similar to that acquired by the bite of sand flies. The majority of children with congenital infection present with the illness within the first year, but the exact means of transmission are not yet completely clear.9–11 Herein we describe two cases of AVL among pregnant patients who transmitted the disease congenitally to their infants admitted between Jan 2007 and Dec 2008 to Dona Regina Hospital, which is a tertiary referral center for maternal and infant care in the city of Palmas, Tocantins, Brazil.
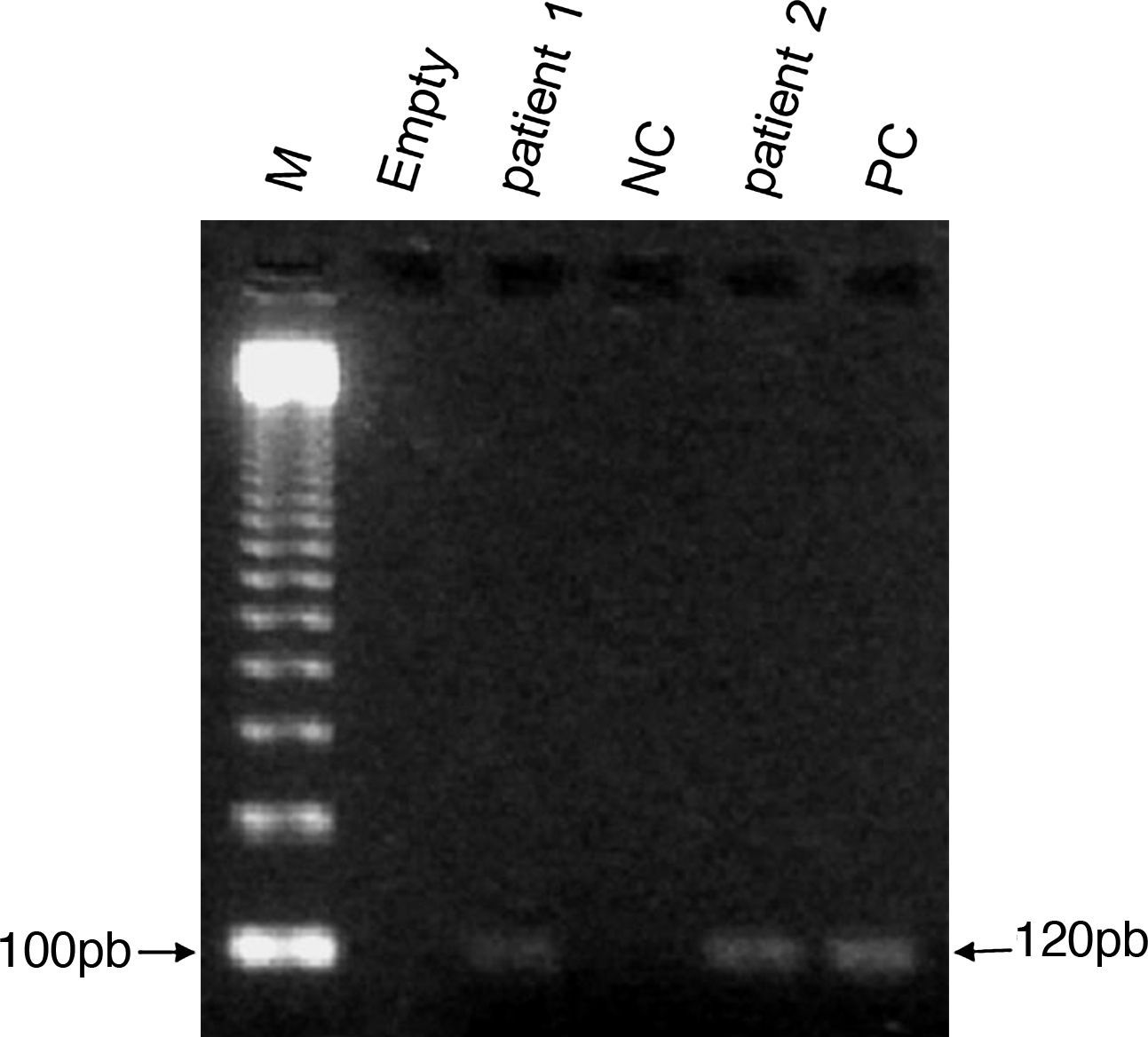
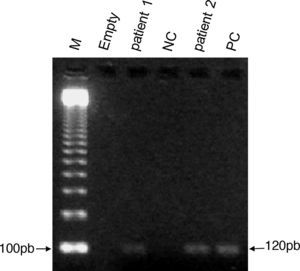
Pregnancy was confirmed by serum levels of β-human chorionic gonadotropin hormone and by an abdominal ultrasound. Indirect fluorescent antibody test (IFAT) test was performed in sera samples to detect antibodies against Leishmania, as recommended by the Brazilian Ministry of Health12; bone marrow smears were stained with Giemsa to detect the presence of Leishmania amastigotes; and a genus-specific polymerase chain reaction (PCR) was performed using bone marrow samples to detect 120bp of the conserved region of parasite kDNA, as reported elsewhere.13 The bone marrow aspirates were obtained from the pregnant women during hospitalization and from the newborns during the first week after their delivery (Fig. 1).
Identification of 120bp of the conserved region of the Leishmania minicircle-kDNA in bone marrow aspirates from newborns using polymerase chain reaction (PCR) revealed in 2% agarose gel stained with ethidium bromide. Lane 1: molecular weight ladder (M); lane 2: empty; lanes 3 and 5: bone marrow from newborns 1 and 2, respectively; lane 4: negative control (NC) without DNA; lane 6: positive control (PC) consisting of cultured promastigotes of Leishmania chagasi (syn. Leishmania infantum).
The study was conducted in agreement with the National Health Council Resolution number 196/96 which regulates research involving human subjects in Brazil and the Helsinki Declaration. A confidentiality agreement was signed by the researchers to protect the patient identity upon obtaining information from their medical records. All patients or their relatives/legal guardians signed a voluntary and informed consent form authorizing the use of their medical records.
Case presentationCase 1A 24-year-old woman from the city of Itacajá, Tocantins, who was pregnant for the second time, was hospitalized with fever above 38°C. During the sixth month of pregnancy she reported fever and weight loss. A physical examination revealed mucocutaneous pallor, dyspnea and slight edema in the lower limbs. Upon review of the abdominal ultrasound it was found a posterior placenta of heterogeneous texture and stage I maturity, a 30-week and 4-day gestational age and a fetal heart rate of 170beats/min, with no evidence of fetal hepatosplenomegaly. Because of acute fetal distress, the patient underwent a cesarean section, resulting in a preterm newborn. The mother presented with productive cough, and the chest X-ray revealed basal infiltration in the right hemi thorax. Antibiotic therapy with intravenous oxacillin and ceftriaxone were initiated and she received a packed red blood cell transfusion. Given the clinical and laboratory evidence (Table 1), the patient underwent a bone marrow aspiration, which revealed the presence of Leishmania amastigotes. Thus, she was started on intravenous amphotericin B desoxycholate 1mg/kg/day. The following day; she had persistent fever, a painful surgical wound, jaundice and echimosis on her abdomen, periorbital region and arms. Then, amphotericin B desoxycholate was replaced by an intravenous liposomal formulation of amphotericin B, 3mg/kg/day, and the patient was transferred to a critical care unit. The patient developed a coagulation disorder, generalized bleeding and died 10 days after being hospitalized. IFAT result obtained on admission was positive with dilution titer of 160 (reference cut-off=40).
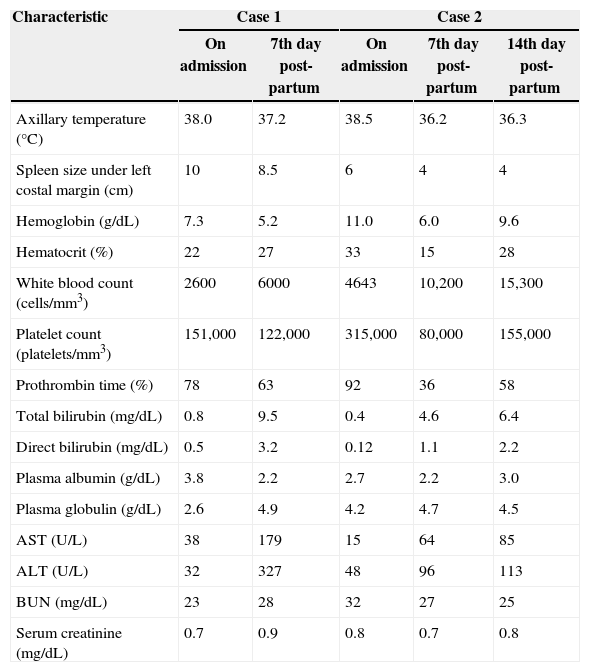
Main clinical and laboratory findings upon hospital admission and during treatment of two pregnant women with American visceral leishmaniasis in Brazil.
| Characteristic | Case 1 | Case 2 | |||
|---|---|---|---|---|---|
| On admission | 7th day post-partum | On admission | 7th day post-partum | 14th day post-partum | |
| Axillary temperature (°C) | 38.0 | 37.2 | 38.5 | 36.2 | 36.3 |
| Spleen size under left costal margin (cm) | 10 | 8.5 | 6 | 4 | 4 |
| Hemoglobin (g/dL) | 7.3 | 5.2 | 11.0 | 6.0 | 9.6 |
| Hematocrit (%) | 22 | 27 | 33 | 15 | 28 |
| White blood count (cells/mm3) | 2600 | 6000 | 4643 | 10,200 | 15,300 |
| Platelet count (platelets/mm3) | 151,000 | 122,000 | 315,000 | 80,000 | 155,000 |
| Prothrombin time (%) | 78 | 63 | 92 | 36 | 58 |
| Total bilirubin (mg/dL) | 0.8 | 9.5 | 0.4 | 4.6 | 6.4 |
| Direct bilirubin (mg/dL) | 0.5 | 3.2 | 0.12 | 1.1 | 2.2 |
| Plasma albumin (g/dL) | 3.8 | 2.2 | 2.7 | 2.2 | 3.0 |
| Plasma globulin (g/dL) | 2.6 | 4.9 | 4.2 | 4.7 | 4.5 |
| AST (U/L) | 38 | 179 | 15 | 64 | 85 |
| ALT (U/L) | 32 | 327 | 48 | 96 | 113 |
| BUN (mg/dL) | 23 | 28 | 32 | 27 | 25 |
| Serum creatinine (mg/dL) | 0.7 | 0.9 | 0.8 | 0.7 | 0.8 |
The newborn female weighed 1170g and was classified as extremely premature. Her weight was appropriate for the estimated gestational age, and she had an Apgar score of 6 and 8 during the first and fifth minutes after birth, respectively. Within the next 24h, she developed grade II pulmonary hyaline membrane disease, for which she was maintained on nasal continuous positive airflow pressure. Splenomegaly and hepatomegaly were detected on physical examination. The following day, the newborn's tachypnea worsened, and she was placed on mechanical ventilation. In addition, she was started on antimicrobial therapy with ampicillin and amikacin. After 72h, the patient developed jaundice and worsening anemia. Bone marrow aspirate smears looking for the presence of Leishmania was negative. Despite multiple blood transfusions, the newborn's erythrocyte indexes continued to decrease. We then performed a PCR test to detect parasite kDNA using the cells collected from the bone marrow aspirate which gave a positive result for Leishmania spp. The newborn was started on treatment with intravenous amphotericin B deoxycholate, 1mg/kg/day for 14 days making satisfactory progress and being discharged from the critical care unit 20 days after birth.
Case 2A 23-year-old woman from the city of Porto Nacional, Tocantins, who was pregnant for the second time, was hospitalized with complaint of intermittent fever during the past 4 months associated with weakness, fatigue, vomiting and anorexia. A physical examination of the patient revealed dehydration, weight loss and edema of the legs. She reported having suffered from eclampsia during her previous pregnancy, and she had undergone five prenatal evaluations with no abnormal blood pressure levels. During the seventh month of pregnancy, she presented with daily fever, lower limb edema, jaundice and progressive anemia. An ultrasound test revealed a slight decrease in amniotic fluid, a posterior placenta of heterogeneous texture and stage II maturity consistent with a 35-week and 4-day gestational age, a fetal heart rate of 169beats/min, splenomegaly and mild right hydronephrosis. Clinical and laboratory findings are described in Table 1. Because of acute fetal distress, it was performed a cesarean section and delivered a preterm infant with a weight which was appropriate for the gestational age. Direct analysis of bone marrow smears from the mother revealed the presence of Leishmania amastigotes. The mother was treated with intravenous liposomal amphotericin B, 3mg/kg/day, IV for 7 days. However, her general condition deteriorated, as indicated by a worsening of her anemia, dyspnea, toxemia and postural hypotension and decrease of prothrombin activity. The patient was transferred to the intensive care unit where she recovered and was discharged after 14 days. IFAT result obtained on admission was positive with dilution titer of 160 (reference cut-off=40).
The newborn male weighed 2590g which was appropriate for the gestational age, and he had an Apgar score of 3 and 5 for the first and fifth minutes of life, respectively. The newborn developed acute respiratory failure due to meconium aspiration and required mechanical ventilation. Splenomegaly was detected on physical examination. The newborn was transferred to the neonatal intensive care unit and was treated with ampicillin and amikacin, with no clinical improvement. Suspecting visceral leishmaniasis, we obtained a bone marrow aspirate looking for Leishmania amastigotes but the results were negative. Due to severe anemia, he required multiple blood transfusions and later, he developed jaundice being started on oxacillin and cefepime plus intravenous liposomal amphotericin B 3mg/kg/day. A PCR test for Leishmania kDNA was positive on bone marrow aspirate. The newborn had satisfactory progress and was discharged 50 days after birth.
DiscussionThe present cases of congenitally acquired visceral leishmaniasis constitute a rare condition. During 2007 and 2008, there were 873 visceral leishmaniasis cases in adults and children residents in the State of Tocantins that were registered in the Brazilian National Surveillance System. Most of the congenital visceral leishmaniasis infections described in the literature follow a course where there are few or no maternal symptoms and where macroscopic changes of the placenta and its annexes are typically not observed. Also, it has not been possible to confirm that transplacental fetal infection has been the mode of transmission in any of the cases that have been reported so far. Eltoum et al. reported a case of a pregnant woman with symptomatic leishmaniasis who suffered a miscarriage.11 The authors proposed that the parasite entered into the fetal circulation during its separation from the placenta or through damaged areas in the maternal–fetal barrier because the mother showed no signs of illness. In addition, laboratory tests on the aborted fetus showed thrombosis, fibrin deposition in a third of the placental chorionic villi, the presence of amastigotes inside and outside macrophages and the absence of an inflammatory reaction in response to the parasites. Unfortunately, placenta histopathologic studies were not performed in the present cases.
In pregnant women, the detection of visceromegaly can be impaired by the growth of the uterus. Among the laboratory studies that pregnant women typically undergo are direct visualization of amastigote forms in bone marrow smears and detection of anti-Leishmania serum antibodies using the IFAT, where the presence of quantifiable titers of antibodies higher than 40-fold dilution of the serum is considered positive. The PCR is a highly sensitive and specific technique to detect Leishmania kDNA, making it an important tool to improve the opportunity of early treatment, especially in newborns to avoid misinterpretation of positive serological tests due to passive transfer of maternal antibodies. The tests performed on the two pregnant women upon their admission revealed that they had high titers of antibodies, which could cross the placental barrier. Although the bone marrow smears were positive for Leishmania amastigotes in the pregnant women, they were not elucidatory in the newborns. PCR amplification of parasite kDNA in newborn's bone marrow samples suggests that leishmaniasis was vertically transmitted because they developed signs of the disease shortly after birth and remained in the hospital until their more severe symptoms were resolved while on specific anti-leishmanial treatment. The early onset of symptoms after birth rules out the possibility that the newborns acquired the disease through vector transmission. This result demonstrates that, using PCR, it is possible to make an early diagnosis of leishmaniasis in the newborn and provide immediate treatment for the mother and fetus to avoid or minimize disease complications.
There is a concern about the effect of the drugs used for the treatment of leishmaniasis on the well-being of the fetus because clinical trials developed for approval purposes with drugs against visceral leishmaniasis usually exclude pregnant women. However, a recent study conducted in Africa showed a higher incidence of abortion in patients treated with pentavalent antimony.14 The transplacental transfer of antimony has been proved in mice, showing that approximately 1/3 of the antimony present in the maternal blood is transferred to the fetus.15 The second woman who was diagnosed benefited from the experience we gained from the first patient who died earlier. As a result, she was treated with liposomal amphotericin B, which could have contributed to her favorable recovery. Because AVL among pregnant women is an extremely serious health concern it is reasonable to recommend treatment with liposomal amphotericin B because it has the best safety profile. Monitoring for the presence of AVL in pregnant women living in endemic areas is essential for the implementation of timely treatment and can prevent fatal outcomes.
Conflict of interestAll authors declare to have no conflict of interest.
We thank the Daniela Carvalho Lemos for technical support. This study had financial support from the Brazilian Ministry of Health (Process: 2007 2029 000137 – MS/CNPq/CECT – N° 01/2006).