To assess the adequacy of medical prescriptions for community-acquired pneumonia at the emergency department of the Hospital de Clínicas de Porto Alegre, we conducted a prospective cohort study, from January through April 2011. All patients with suspected pneumonia were selected from the first prescription of antimicrobials held in the emergency room. Patients with a description of pneumonia, community-acquired pneumonia, respiratory infection, or other issues related to community-acquired pneumonia were selected for review. Two-hundred and fifteen patients were studied. Adherence to the hospital care protocol was: 11.2% for the initial recommended tests (chest X-ray and collection of sputum sample), 34.4% for blood cultures, and 92.1% for the antimicrobial choice. Sixty percent of the prescriptions consisted of a combination of drugs, and the association of beta-lactam and macrolide was the most common. The Hospital Infection Control Committee evaluated patients’ prescriptions within a median time of 23.5h (IQR 25–75%, 8–24). Negative evaluations accounted for 10% of prescriptions (n=59). Fourteen percent of the patients died during hospitalization. In the multivariate analysis, Pneumonia Severity Index Score and use of ampicillin+sulbactam alone were independently related to in-hospital mortality. There was a high adherence to the hospital's CAP protocol, in relation to antimicrobial choice. Severity score and use of ampicillin+sulbactam alone were independently associated to in-hospital death.
Community-acquired pneumonia (CAP) is an acute infection that affects the lower respiratory tract, which occurs in patients outside the hospital or develops within the first 48h after hospital admission.1 Often this diagnosis is made-up by a combination of clinical symptoms of acute respiratory disease, laboratory tests, microbiological findings, response to antimicrobial therapy and especially, by chest X-ray alone, despite the low sensitivity and specificity of this test for pneumonia diagnosis.2
Worldwide incidence of pneumonia is 12cases/1000 inhabitants per year. Pneumonia represents the leading cause of death from infectious disease, despite all the advances in medicine over the years, such as, the availability of new antibiotics and effective vaccines.3 In Brazil, in 2009, of the total admissions for respiratory diseases, 52.0% were for pneumonia, representing 7.3% of total admissions in the country.4
There are several clinical scores designed to stratify patients, regarding severity and mortality due to CAP, including the Pneumonia Score Index (PSI). The PSI assigns points based on age, comorbidities and abnormalities in the physical examination. This score classifies pneumonia patients into five groups, in increasing order of severity and death risk over the next 30 days.1,5 In the Hospital de Clínicas de Porto Alegre (HCPA) there is a clinical care guideline for patients with CAP, based on the PSI, which serves as a decision making tool for processing whether individuals can be managed on an outpatient basis or require hospitalization.6 Besides, an infection control committee evaluates antibiotic prescriptions and is available seven-days a week for antimicrobial consultation.
The objective of this study was to assess the management adequacy of patients with CAP according to local guidelines and recommendations from the hospital infection control committee.
Materials and methodsSettingThe Hospital de Clinicas de Porto Alegre is the teaching hospital of the Universidade Federal do Rio Grande do Sul (UFRGS). With 795 beds for 67 medical specialties, it provides assistance, education and health research. The hospital has an adult public emergency department with 49 beds that receives patients 24h a day. Patients treated in the emergency department are predominantly from Porto Alegre (56.0%), from the surrounding area (32.0%), and from other parts of the state (12.0%).
The HCPA has an infection control committee (ICC) composed of six physicians, four nurses, one pharmacist, two resident pharmacists, two resident nurses, as well as 10 trainees who are nursing and pharmacy graduates fellows. In the hospital's antimicrobial panel, 70% of the selected antimicrobials need a physician filled order for prescription, which are then analyzed by the ICC. The ICC's evaluations are conducted Monday through Friday during business hours.
Study design and definitionsWe conducted a prospective cohort study of patients with CAP from January 20th until April 20th, 2011. All patients were selected from the antibiotic order forms, included in the system by the emergency department. Patients with suspected pneumonia, CAP, respiratory infection, or any other justification related to CAP were included for review.
The study included adult patients, over 18 years of age, without immuno-suppression (i.e. transplanted patients or HIV/AIDS patients), admitted in less than 48h to the emergency department and, had not used antimicrobials prior to admission.
Patients were selected according to the antimicrobial order forms included in the institution's database. This way, patients taking antibiotics not evaluated by the ICC team, such as amoxicillin, ampicillin, doxycycline, and erythromycin, were not included in the study.
We reviewed the demographic characteristics of the patients, age and gender, previous comorbidities, antimicrobial choice, evaluations conducted by the ICC team, PSI classification, and adequacy of prescription, according to local care guidelines.
Adherence to the care protocol was stratified into three steps: step 1 – request for a chest X-ray and sputum analysis; step 2: request for blood culture; step 3: antimicrobial choice.
The Infection Control Committee evaluations were divided into: positive evaluations – prescription approved; prescription approved with restriction of time; approved until culture results; recommendation for route change; and evaluations considered negative – not approved, pending further information, and recommendation for changing antimicrobial choice.
All enrolled patients were followed-up at the hospital until the outcome: either discharge from the institution or death.
This investigation was conducted in accordance with the “Guidelines and Norms Regulating Research Involving Human Subjects” adopted by the National Health Council in its Resolution 196/96 and approved by the Ethics Committee of Research of the HCPA, accredited by the National Commission on Research Ethics under number 10-0468.
Statistical analysisTo determine the distribution pattern of the variables, the Kolmogorov–Smirnov test was used. Variables with skewed distribution were presented as medians and interquartile ranges (IQR). Differences between variables were expressed as odds ratios and 95% confidence intervals. A p<0.05 value was considered statistically significant. To investigate the relationship between specific variables and the in-hospital death outcome, logistic regression analysis was performed using backward step method. The data were compiled in Microsoft Excel® and analyzed with the Statistical Package for Social Science (SPSS), version 18.0.
ResultsTwo hundred and seventy-eight patients were included. Of these, 58 patients presented changes to the initial diagnosis of CAP: urinary tract infection, asthma, or bronchitis, and were, therefore, excluded from the study. From the 220 remaining patients, there was a loss to follow-up of 5 patients; four due to desertion from the emergency department, and one patient's data were lost in the database. Therefore, we included 215 patients in the final analysis.
Patients’ demographic characteristics are shown in Table 1.
Demographic characteristics and comorbidities of patients with community-acquired pneumonia at the HCPA, January–April 2011.
| Patients | n=215 |
|---|---|
| Age (median, IQR) | 67 (58–77) |
| 60 years old or more | 155 (72.1) |
| Female | 119 (55.3) |
| Comorbidities | |
| Cardiovascular disease | 110 (51.2) |
| Diabetes | 54 (25.1) |
| COPD | 53 (24.7) |
| Cancer | 45 (20.9) |
| Severity score | |
| PSI 1 | 15 (7) |
| PSI 2 | 24 (11.2) |
| PSI 3 | 54 (25.1) |
| PSI 4 | 108 (50.2) |
| PSI 5 | 14 (6.5) |
| Hospitalization days (median, IQR) | 8 (4–17) |
Data are n (%) of patients, unless otherwise indicated. IQR, interquartile range; COPD, chronic obstructive pulmonary disease.
Adherence to the local care guidelines was 11.2% for step 1, 34.4% for step 2, 92.1% for step 3, and 1.9% for the full guideline. All patients (n=215) had a chest X-ray requested, 37.7% (n=81) blood culture, 25.1% (n=54) microbiology of sputum, and 13.5% (n=29) had the acid-fast bacilli exam requested. Out of 81 blood cultures, 10 (12.3%) were positive. Among the microorganisms found, the most prevalent were: Streptococcus pneumoniae (n=3), Klebsiella pneumoniae (n=2), Gram positive bacilli (n=1), Escherichia coli (n=1), Pseudomonas aeruginosa (n=1), Enterococcus spp. (n=1) and Staphylococcus aureus (n=1). In the sputum culture results, there were six outstanding strains: one P. aeruginosa, one S. aureus, one S. pneumoniae, one Klebsiella spp. and two Candida spp.
All patients underwent a diagnostic X-ray. Radiological changes in 47.4% (n=102) of the patients showed consolidation, in 19.5% (n=42) infiltrates, in 6.5% (n=14) pleural effusion, and in 8.4% (n=18) other results. In 9.3% (n=20) of cases, the X-ray was normal, and in 8.8% (n=19) there were no results reported in the system.
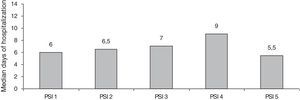
The distribution of patients, by risk stratification, according to the PSI score was: PSI 1 (7%, n=15), PSI 2 (11.2%, n=24), PSI 3 (25.1%, n=54), PSI 4 (50.2%, n=108) and PSI 5 (6.5%, n=14). The median hospital stay was 8 days (IQR 25–75%, 4–17) and their stratification, according to the severity score index, is shown in Fig. 1.
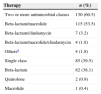
The antibiotics chosen as first-line therapy were the combination of cefuroxime and azithromycin in 40% (n=86) of cases, followed by ampicillin+sulbactam in 12.6% (n=27), cefuroxime in 10.7% (n=23) and a combination of ampicillin+sulbactam plus azithromycin in 4.7% (n=10). Sixty percent (n=130) of patients were treated with a combination of two or more drugs (Table 2).
Antimicrobial therapy in patients with community-acquired pneumonia at the HCPA, January–April 2011.
| Therapy | n (%) |
|---|---|
| Two or more antimicrobial classes | 130 (60.5) |
| Beta-lactam/macrolide | 115 (53.5) |
| Beta-lactam/clindamycin | 7 (3.2) |
| Beta-lactam/macrolide/clindamycin | 4 (1.8) |
| Othersa | 4 (1.8) |
| Single class | 85 (39.5) |
| Beta-lactam | 82 (38.1) |
| Quinolone | 2 (0.9) |
| Macrolide | 1 (0.4) |
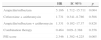
The ICC made a median of three evaluations per patient, corresponding to 596 recommendations for the first antimicrobial adopted. The median time, from prescription until evaluation, was 23.5h (IQR 25–75%, 8–24). Negative evaluations accounted for 9.8% (n=59) of the first antimicrobial prescribed. The median length of time of antimicrobial use, approved by the ICC team, was 3 days (IQR 25–75%, 3–5) – Table 3.
Infection Control Committee evaluation of the first antimicrobial prescription in patients with community-acquired pneumonia at the HCPA, January–April 2011.
| Overall ICC evaluations | 597 (100) |
| Positive evaluations | 538 (90.2) |
| Approved | 240 (40.2) |
| Approved with restriction of time | 77 (12.8) |
| Approved until culture results | 122 (20.4) |
| Switch to oral therapy | 99 (16.5) |
| Negative evaluations | 59 (9.8) |
| More information needed | 32 (5.3) |
| Another option suggested | 23 (3.8) |
| Not approved | 4 (0.6) |
| Time for evaluation in hours (median, IQR) | 23.5 (8–24) |
| Number of evaluations per patient (median, IQR) | 3 (2–4) |
| Length of time approved, in days (median, IQR) | 3 (3–5) |
IQR, interquartile range.
Data are n (%) of ICC evaluations, unless otherwise indicated.
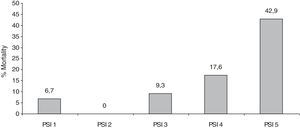
Fourteen percent of patients (n=31) died during hospitalization. Of these, 38% had cancer and 38% suffered from cardiovascular disease. The association between mortality and the severity score is shown in Fig. 2.
In multivariate analysis, the severity score and the use of ampicillin+sulbactam were independent predictors of in-hospital mortality (Table 4). Variables selected for multivariate analysis, based on the significance on univariate analysis were: use of ampicillin+sulbactam, cefuroxime+azithromycin, ampicillin+sulbactam+azithromycin, an association of two or more classes of antimicrobials and the PSI score.
Multivariate risk analysis of factors associated with in-hospital mortality in patients with community-acquired pneumonia at the HCPA, January–April 2011.
| HR | IC 95% | p | |
|---|---|---|---|
| Ampicilin/sulbactam | 5.186 | 1.712–15.711 | 0.004 |
| Cefuroxime+azithromycin | 1.731 | 0.341–8.786 | 0.508 |
| Ampicilin/sulbactam+azithromycin | 1.331 | 0.102–17.37 | 0.828 |
| Combination therapy | 0.464 | 0.09–2.388 | 0.358 |
| PSI score | 2.346 | 1.302–4.225 | 0.005 |
Variables selected for multivariate analysis were those with p<0.05 in univariate analysis: use of ampicillin+sulbactam, cefuroxime+azithromycin, ampicillin+sulbactam+azithromycin, an association of two or more antimicrobial classes and the PSI severity score.
There was poor adherence to local guidelines except for antimicrobial choice. Most patients had high PSI. The ICC team's evaluation, related to antimicrobial choice, was mostly positive, and the treatment of choice, with two or more classes of antimicrobials, prevailed. The in-hospital mortality was 14%, similar to other centers. Ampicillin+sulbactam and high PSI scores were independently related to in-hospital mortality.
In a prospective study involving patients who were hospitalized for CAP in Brazil the average patients age was 65 and patients more than 60 years old accounted for 67% of the sample.7 In the United States, an observational study showed that 59% of hospitalized patients with CAP, were 65 years old or more. Cardiovascular diseases (43%) and respiratory illnesses (34%) were the most prevalent comorbidities.8
In our study, the PSI indicates a population that required hospitalization: brief hospitalization in 25% (PSI 3), prolonged hospitalization in 56% (PSI 4 an 5). On the other hand, patients classified as PSI 1 and 2, who should be discharged, were treated as in-patients 90% of the time, with a median stay of around 6–7 days. In a study at the HCPA in 2005, 24% of the patients who attended the emergency for CAP had a PSI score of 3, 29% had a PSI of 4, and 14% had a PSI of 5. In this study, 70% of the population with a PSI of 1 or 2 was treated as in-patients.9 A prospective study in Chile, in immunocompetent patients with CAP, 23% had a PSI of 3, 38% PSI 4, and 17% had a PSI of 5.10 Our results show a greater proportion of patients with increased mortality risk (PSI 4 and 5), indicating a need for long hospital stays. However, most patients with low risk scores were treated as in-patients, increasing the risks related to hospital infection to these patients, costs to the health system, and overcrowding of the emergency department. The reasons for these hospitalizations were not reviewed.
The rate of adherence to local guidelines regarding blood cultures, recommended by international guidelines, was low. However blood culture tests, when requested, obtained negative results in most cases, and when a microorganism was identified it did not change the patient's therapy. Moreover, in spite of the low rate of positive culture results, in both blood and sputum cultures, there was no relation between the bacteria identified in sputum and those identified in blood cultures.
In a study of CAP patients, blood cultures were positive in less than 20% of cases.11 A Canadian study found that the contribution of blood cultures for clinical management of patients with CAP was low. Only 5% of blood cultures were positive in immunocompetent patients and the clinical evaluation was more decisive than the test results to determine treatment.12 The Brazilian guidelines recommend that blood cultures should be collected in critically ill patients who do not respond to antimicrobial therapy.1 Although most international guidelines recommend this test for in-patient management, based on our results, we must review this recommendation in our local guidelines.
Adherence to clinical guidelines is a difficult task. Adherence to the recommended chest X-ray examination and antimicrobial choice was high. The X-ray is essential for the diagnosis of pneumonia, helps in the severity assessment,1 and it is easy to perform in our setting. The high adherence to antimicrobial choice recommended by the HCPA is a reflection of the ICC team's work. The ICC team evaluates more than 150 prescriptions daily. Therefore the ICC continuously reinforces local protocols recommendations.
The mortality of patients hospitalized for CAP can vary from 5.7 to 14%.13 The mortality associated with the severity score in our study was greater than that in the study that validated the PSI. In this study risk of death for PSI Classes 4 and 5 were 8% and 29%, respectively.5 In our study 17% of patients in the PSI 4 score group died, and 42% of patients in the PSI 5 class, died during hospital admission.
For empirical antimicrobial therapy, national and international guidelines recommend the use of a combination therapy with a beta-lactam and a macrolide or fluroquinolone for patients requiring hospitalization.1,3,14 A study evaluating the impact of therapy on mortality suggests superiority of beta-lactam antibiotics associated with a macrolide compared with beta-lactam monotherapy on 14-day mortality in patients with severe CAP.15 Our study demonstrates the predominant use of empirical therapy with an association between beta-lactam antibiotics and macrolides, with a higher mortality in patients who received single therapy of ampicillin+sulbactam in the logistic regression analysis, which is in accordance with national and international guidelines.
Our study has limitations. This is a single center study, which limits the generalization of the results. Patients who used antimicrobials that were not evaluated by the IC team were not included. Factors that may be related to mortality, such as the timing for initiation of antimicrobial therapy or dose of antibiotics prescribed, were not evaluated. However, the results of high adherence to prescription recommendations, in response to daily IC team evaluations, can serve as a model of the protocol's successful management. Protocol recommendations must be systematically reinforced to guarantee physicians compliance.
In conclusion, most patients with pneumonia were classified as having moderate to severe infections. High adherence to the Protocol was found regarding antimicrobial choice, and X-ray performance, but poor adherence to blood culture exams. The use of ampicillin+sulbactam, without a macrolide, was associated with higher mortality. There is a need for a pneumonia protocol review in our setting and this work might contribute to changes in this local protocol. We stress the importance of local data to guide decision-making recommending protocols.
Conflict of interestThe authors declare no conflict of interest.