Limited information is available regarding AmpC β-lactamase (ABL)-producing Enterobacteriaceae compared to extended-spectrum β-lactamase-producing enterobacteria. Since ABL-producing organisms are often resistant to multiple antimicrobial agents, therapeutic options against these pathogens are limited. Among 230 clinical Enterobacteriaceae isolates, 64 (27.8%) were found to produce ABL in our study. Escherichia coli (83.9%) was a predominant pathogen, followed by Citrobacter freundii (5.2%). A significant proportion of ABL-producing isolates (81.3%) were found to be multidrug resistant against commonly used antibiotics. Univariate analysis showed that prior history of taking antibiotics (odds ratio [OR], 5.278; confidence interval [CI], 2.838–9.817; p<0.001) and being inpatients (OR, 4.587; CI, 2.132–9.9; p<0.001) were associated with ABL positivity. Regular antimicrobial resistance surveillance for ABL-producing Enterobacteriaceae is warranted for proper antimicrobial treatment strategy and policy making due to ABL-positive infections.
The emergence of AmpC β-lactamase (ABL)-producing Enterobacteriaceae is now a major worldwide problem due to their features of non-beta-lactam coresistance and high potential to transfer the drug resistance features horizontally to other Enterobacteriaceae members via their transmissible plasmid(s).1 In recent years, there are increasing reports about the dissemination of ABL-producing Enterobacteriaceae from hospital to community in different countries. However, little is known about the occurrence of ABL-producing Enterobacteriaceae in Nepal, although high rates of prevalence have been reported in neighbouring countries, up to 36.5% in India,2 35.6% in Pakistan3 and 26.2% in China.4 We conducted a prospective study in a tertiary care hospital of Nepal, located at capital Kathmandu, with the aim to determine the prevalence, etiology and antimicrobial susceptibility of ABL-producing Enterobacteriaceae (other than typhoidal salmonella), and the conjugational transferability of ABL-positive phenotypes in Escherichia coli (E. coli). Additionally, the present study also highlights the clinical characteristics of patients infected with ABL-positive Enterobacteriaceae and identified some of the risk factors for ABL-positive infections.
During a six-month period of 2007, a total of 230 consecutive isolates of Enterobacteriaceae from the same number of non-repeat patients were collected by Kathmandu Model Hospital Laboratory from different clinical specimens, including urine, swabs and fluids. All suspected symptomatic patients with culture-proven diagnosis of Enterobacteriaceae for the collected samples from the suspected anatomical sites of infections were considered to acquire Enterobacteriaceae infections. Each of the included 230 patients was interviewed directly using a structured questionnaire to collect the data about patient's demographics, patient's category (outpatient or inpatient), prior antibiotic exposure, history of urinary tract infection (UTI) and presence of any underlying illnesses (diabetes mellitus, malignancies, liver disease and renal dysfunction) or comorbidities (recent surgical operations in the last six months, taking immunosuppressant, using indwelling urinary catheter or other mechanical devices). Written informed consents were obtained from all patients prior to inclusion in the study. This study was approved by the Ethics and Research Committee of Kathmandu Model Hospital.
All the received clinical samples were collected and processed by standard microbiological methods.5 Following collection the sample was transferred to the laboratory immediately and inoculated on blood agar and McConkey agar. Isolates were identified based upon colonial characteristics and conventional biochemical tests.5 Antimicrobial susceptibility patterns were determined by disk diffusion method using commercial antibiotic disks (Oxoid Ltd., Basingstoke, UK).6 Identification of isolates of Enterobacter cloacae, Enterobacter aerogenes and Citrobacter spp. were judged as ABL producers, as they are known for chromosomal ABL producers and often produce ABL constitutively.1,7 The screening for other presumptive ABL-producers in the possibility of presence of extended-spectrum β-lactamase (ESBL) was improved by the criteria of cefoxitin (30μg) insusceptibility, positivity for ESBL screening and negative result for ESBL confirmation. For the assessment of ESBL production, double-disk synergy test using a disk containing of amoxicillin/clavulanic acid (20/10μg) was performed using positive and negative control strains for ESBL production.8 The phenotypic confirmation of ABL-positive was determined by AmpC disk test.9 The AmpC disk containing a 1:1 mixture of saline and 100× Tris–EDTA solution was made in-house according to Black et al.9 The procedure of AmpC disk testing was followed according to Black et al.9 In brief, a cefoxitin disk (30μg) was placed on the lawn of E. coli ATCC 25922 on Mueller-Hinton agar plate. Just before the use, AmpC disk was moistened with 20μL of saline and several colonies of a test organism were applied to a disk and then placed to almost touching a cefoxitin disk in inoculated surface of agar with the face of inoculated disk in contact with the agar surface. After incubation overnight, the ABL production was identified by the presence of either an indentation or a flattening of the zone of inhibition. We excluded the isolates of Salmonella enterica serotype Typhi and Paratyphi A because none of them were found resistant to 2nd and 3rd generation cephalosporins at the time of study, and it was felt that including them could misestimate the prevalence of ABL-producing isolates among Enterobacteriaceae.
The ABL-positive E. coli isolates which were susceptible for streptomycin were screened and selected for conjugation study to evaluate the horizontal transfer of ABL-positive phenotypes. Evidence of the similar size of intact plasmid(s) between donors and transconjugants, the resistant features to cefoxitin, cefatzidime (30μg), cephotaxime (30μg) and amoxicillin/clavulanic acid by transconjugants, and the positive result of AmpC disk test by transconjugants was judged as transferable ABL-positive phenotypes. The plasmid was extracted and purified using commercial kits (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. Conjugation was done as described by Baral et al.,10 using plasmid free E. coli HB 101 (F−S+lac−) as the recipient strain. Transconjugants were selected on MacConkey agar supplemented with streptomycin (100μg/mL) and cefoxitin (20μg/mL).
Statistical analyses were performed using SPSS (version 18.0, SPSS Inc., Chicago, IL) software package. Categorical variables were analyzed by Chi-square test or Fisher's exact test, as appropriate. Univariate analysis was performed for calculation of odds ratio (OR) with 95% confidence interval (CI) for potential risk factors. A p-value of less than 0.05 was considered statistically significant.
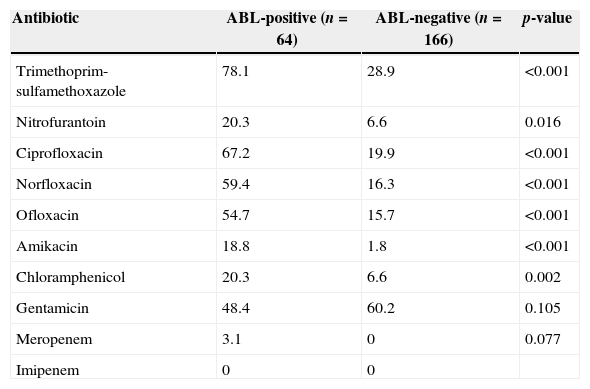
A total of 230 Enterobacteriaceae isolates were recovered from 1, 503 clinical specimens during 6-month study period. Out of the total isolates, E. coli (83.9%) was the predominant pathogen, followed by Citrobacter freundii (5.2%) and Enterobacter spp. (3%). Totally 64 (27.8%) ABL-producing Enterobacteriaceae were observed in 230 Enterobacteriaceae isolates, among them E. coli (59.7%) was the most common organism, followed by C. freundii (18.8%). The mean age of the patients harbouring ABL-producing Enterobacteriaceae was 49.3 years (range 2–92 years). None of the isolates of Proteus spp. and Klebsiella spp. were shown to produce ABL in our study. Similar to our study, high occurrence rates of ABL-producing Enterobacteriaceae were reported in other Asian countries.2,4,11 The majority of ABL-producers were found to exhibit high rates of resistance to commonly used antibiotics as shown in Table 1. Specifically, high rates of resistance observed to fluoroquinolones (67.2% resistance to ciprofloxacin, 59.4% to norfloxacin, 54.7% to ofloxacin), aminoglycosides (48.4% resistance to gentamicin) and nitrofurantoin (20.3% resistance) are most worrisome (Table 1), because these drugs can be bought even by low-income class of Nepalese people and are easily available in different pharmacy counters across Nepal. Two ABL-producing C. freundii were found resistant to meropenem, while none of the isolates was found resistant to imipenem. It is not uncommon to find carbapenem resistance among ABL-producing isolates.12 Comparatively, rate of meropenem insusceptibility (0.87% resistance in Enterobacteriaceae) observed in our study is lower than a report from India (22.16% resistance among Gram-negative bacterial pathogens).13 As we expected, a significant proportion of ABL-producing isolates (81.3%) showed resistance to ≥3 different classes of antibiotics, defined as multidrug resistant (MDR).
Antimicrobial resistance (%) by AmpC β-lactamase (ABL)-positive and -negative Enterobacteriaceae isolates.
| Antibiotic | ABL-positive (n=64) | ABL-negative (n=166) | p-value |
|---|---|---|---|
| Trimethoprim-sulfamethoxazole | 78.1 | 28.9 | <0.001 |
| Nitrofurantoin | 20.3 | 6.6 | 0.016 |
| Ciprofloxacin | 67.2 | 19.9 | <0.001 |
| Norfloxacin | 59.4 | 16.3 | <0.001 |
| Ofloxacin | 54.7 | 15.7 | <0.001 |
| Amikacin | 18.8 | 1.8 | <0.001 |
| Chloramphenicol | 20.3 | 6.6 | 0.002 |
| Gentamicin | 48.4 | 60.2 | 0.105 |
| Meropenem | 3.1 | 0 | 0.077 |
| Imipenem | 0 | 0 |
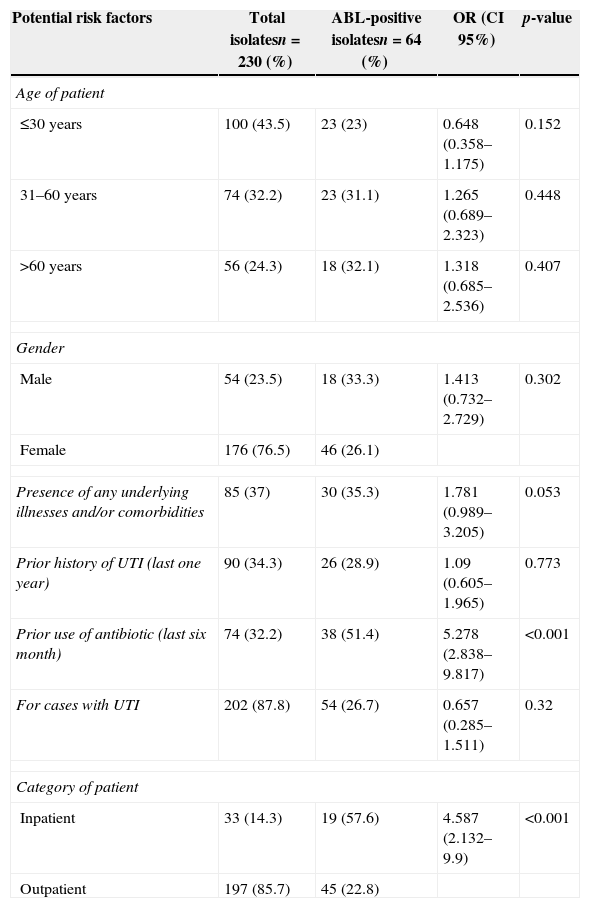
Out of 33 ABL-producing E. coli isolates, 72.7% (n=24) exhibited transferable ABL-positive phenotypes as revealed by conjugation study. The majority of ABL-producing donor isolates harbored multiple plasmids most commonly of size 32.5kb and 38kb, whereas plasmid of size 32.5kb was the most common plasmid among the ABL-producing transconjugants. ABL positivity with the MDR pattern (amoxicillin-, ciprofloxacin-, cefixime-, trimethoprim-sulfamethoxazole-, ofloxacin-, and norfloxacin-resistance) was found most commonly (78%) to be transferred to recipient strain by conjugation. The high prevalence rate of transferable ABL-positive phenotypes observed in our study may indicate an increased transmission rate of drug resistant plasmids that have acquired genes for AmpC enzymes among pathogenic Enterobacteriaceae isolates, which can consequently make the bacteria which are known for lacking or poorly expressing chromosomal AmpC β-lactamase gene, such as in E. coli and Klebsiella pneumoniae.1 The clinical characteristics of 230 patients infected with Enterobacteriaceae are shown in Table 2. Univariate analysis revealed that prior use of antibiotic in the last six months (OR, 5.278; CI, 2.838–9.817; p<0.001) and being inpatient (OR, 4.587; CI, 2.132–9.9; p<0.001) were associated with ABL-positive infections (Table 2). It is not surprising to mention that in Nepal there is extensive practice of irrational antibiotic use, especially by self-medication because drugs are easily accessible in the pharmacy counters without prescription, and this may create the selection pressure to emerge ABL-positive MDR pathogens as we observed in the present study. However, further molecular study involving the isolates from different regions of Nepal is needed to confirm such situation in Nepal. The high frequency of ABL-producing Enterobacteriaceae infections (57.6%) among the inpatients implies the extensive dissemination of ABL-producing bacteria in hospital environments (Table 2). An increased rate of occurrence of ABL-producers (63.4%) was also observed among nosocomial isolates of E. coli and K. pneumoniae in a recent report from India.14 It may indirectly suggest that the contributors that led to the emergence and spread of ABL-producing Enterobacteriaceae among the hospitalized patients of both countries have a common mechanism.
Univariate analysis of AmpC β-lactamase (ABL) positivity among pathogenic Enterobacteriaceae isolates.
| Potential risk factors | Total isolatesn=230 (%) | ABL-positive isolatesn=64 (%) | OR (CI 95%) | p-value |
|---|---|---|---|---|
| Age of patient | ||||
| ≤30 years | 100 (43.5) | 23 (23) | 0.648 (0.358–1.175) | 0.152 |
| 31–60 years | 74 (32.2) | 23 (31.1) | 1.265 (0.689–2.323) | 0.448 |
| >60 years | 56 (24.3) | 18 (32.1) | 1.318 (0.685–2.536) | 0.407 |
| Gender | ||||
| Male | 54 (23.5) | 18 (33.3) | 1.413 (0.732–2.729) | 0.302 |
| Female | 176 (76.5) | 46 (26.1) | ||
| Presence of any underlying illnesses and/or comorbidities | 85 (37) | 30 (35.3) | 1.781 (0.989–3.205) | 0.053 |
| Prior history of UTI (last one year) | 90 (34.3) | 26 (28.9) | 1.09 (0.605–1.965) | 0.773 |
| Prior use of antibiotic (last six month) | 74 (32.2) | 38 (51.4) | 5.278 (2.838–9.817) | <0.001 |
| For cases with UTI | 202 (87.8) | 54 (26.7) | 0.657 (0.285–1.511) | 0.32 |
| Category of patient | ||||
| Inpatient | 33 (14.3) | 19 (57.6) | 4.587 (2.132–9.9) | <0.001 |
| Outpatient | 197 (85.7) | 45 (22.8) | ||
UTI, urinary tract infection.
In conclusion, we observed a high prevalence rate of ABL-producing Enterobacteriaceae among Nepalese patients and the majority of them showed increased rates of antibiotic resistance. To our knowledge, this is among the first reports to describe about clinical and microbiological characteristics of ABL-producing Enterobacteriaceae, and documenting plasmid-mediated transfer of ABL-positive phenotypes of E. coli from clinical isolates of Nepal. Our study not only strongly suggests the dissemination of ABL-producing Enterobacteriaceae in our locality, but also among the patients of all geographical locations of Nepal, because many people from outside of Kathmandu regularly visit our hospital seeking for better treatment facility. Regular nationwide antimicrobial resistance surveillance incorporating the detection of ABLs is warranted in order to restrict the possible burden associated with ABL-positive Enterobacteriaceae infections.
Conflict of interestAll authors declare to have no conflict of interest.