Recent studies suggest that sustained use of generic antibiotics may be associated with clinical failure and emergence of antibacterial resistance. The present study was designed to determine the clinical outcome between the use of generic meropenem (GM) and brand-name meropenem (BNM). Additionally, this study evaluated the economic impact of GM and BNM to determine if the former represents a cost-effective alternative to the latter.
MethodsPatients treated between January 2011 and May 2014 received GM while patients treated between June 2014 and March 2017 received BNM. Mortality was compared between groups. Total infection cost was defined by the cost of antimicrobial consumption, length of stay, and laboratory and imaging exams until infection resolution.
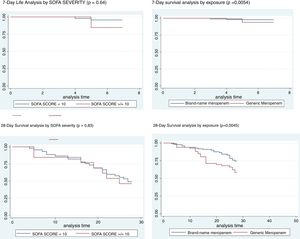
FindingsA total of 168 patients were included; survival rate for the 68 patients treated with GM was 38% compared to 59% in the patients treated with BNM. Multivariate analysis showed that the variables most strongly-associated with mortality were cardiovascular disease (OR 18.18, 95% CI 1.25–262.3, p = 0.033) and treatment with generic meropenem (OR 18.45, 95% CI 1.45–232.32, p = 0.024). On the other hand, total infection cost did not show a significant difference between groups (BNM $10,771 vs. GM $11,343; p = 0.91).
InterpretationThe present study suggests that patients treated with GM have a risk of death 18 times higher compared to those treated with BNM. Furthermore, economic analysis shows that GM is not more cost effective than BNM.
SummaryMore studies measuring clinical outcomes are needed to confirm the clinical equivalence of brand-name versus generic antibiotics, not only for meropenem but also for other molecules.
The generic medication contains the same components and specifications as the brand-name medication. In Colombia, generic medications are available for purchase 10 years after the commercial patent of the original brand-name medication expires. Generic medications must comply with a variety of conditions to be approved for commercial use. Broadly speaking, generic medications must contain the same quantitative and qualitative composition of active ingredients compared to their brand-name counterpart. Furthermore, the pharmaceutical presentation must be similar to their respective brand-name counterpart with regards to formulations (e.g., parenteral, enteral, etc.), safety profile, and pharmacokinetics.1 The prescription of generic medications is considered one of the most effective approaches for reducing cost and increasing global access to first-line medication for the treatment of diverse infections.2,3
Many countries have progressively opened their markets to generic medications. In 2010, generic antibiotics (GA) represented more than two-thirds of the global consumption of medications.3 In the United States, generic medications made up to 63% of total prescriptions in 2007,4 while in England that number was 83% in 2008.5 The generic medication market has likewise expanded in other developed countries such as Japan.2
Recently, the use of generic medications has caused controversy, particularly in the case of GA. It has traditionally been considered that GAs, principally in parental form, have the same efficacy as brand-name antibiotics (BNA).2,3,6–9 However, several studies have questioned the clinical effectiveness of GAs, reporting issues like clinical failure or emergence of bacterial resistance associated with the sustained use of Gas.3,5,7,8
Antibiotic resistance has also been related to a significant use of GAs following their release into the drug market. This collateral damage has been demonstrated in Germany and Denmark where use of generic quinolones generated a disproportionate increase in its use, followed by an increase in resistance levels from 7.7% to 25% and 0.8% to 4%, respectively.10,11 This phenomenon has also been reported in Colombia where the use of β-lactams has surpassed the 90th percentile of the defined daily dose, considerably more than has been reported in other countries.12–14 On a national level, some antibiotics are consumed more than others, as is the case with meropenem given the ready availability of its generic version. Meropenem provides adequate coverage against Pseudomonas aeruginosa and is considered first-line treatment for a number of complicated infections including intra-abdominal infections, bacterial meningitis, blood stream infections, nosocomial pneumonia, septicemia, and febrile neutropenia.15
On the other hand, the characteristics of GAs can be affected by a variety of elements, such as active ingredient, bioequivalence, impurities, additives, and excipients. Many of these elements depend on the manufacturing process, which can vary among GAs and BNAs. In a country where GAs represent a large portion of medications consumed due to limited resources, the therapeutic efficacy of GAs needs to be evaluated. This will contribute to greater confidence in the prescription of GAs, as well as provide a better understanding of possible deleterious effects related with their use.
Keeping in mind the current uncertainties regarding possible differences between GAs and BNAs, this study was designed to determine the clinical impact associated with the use of generic versus brand-name meropenem for the treatment of infections caused by Gram-negative bacteria susceptible to carbapenems. This study evaluated patients who were hospitalized in the intensive care unit (ICU) of a tertiary care hospital in Colombia.
Materials and methodsStudy designThis cohort study compared patients who received generic and brand-name meropenem. The group that received generic meropenem included patients hospitalized in the ICU between January 2011 and May 2014 in a tertiary care hospital in Pereira, Colombia. The introduction of the generic was part of the hospital administration strategy to reduce costs. In contrast, patients hospitalized from June 2014 to March, 2017 in the same ICU were treated with brand-name meropenem based on a new policy of buying brand antibiotics again; these patients constitute the brand-name cohort.
During both time periods of the study, the infection control surveillance did not change nor the prevention strategies implemented by the Infectious Disease Committee. Also, the dosing, infusion and time of treatment for meropenem was the same based on the institutional antimicrobial guidelines in both groups.
Inclusion criteria for the study were age over 18 years, patients hospitalized in the ICU, an infection caused by meropenem-susceptible Gram-negative bacteria, and treatment with meropenem. Patients were excluded if any of the following criteria were met: death within the first 72 h of initiation of meropenem therapy, a concomitant invasive fungal infection, concomitant prescription of four or more doses of antibiotics with the same antibacterial activity as meropenem, and incomplete patient records.
For sample size calculation, mortality in Colombian ICUs associated to intrahospital infections was assumed to be 25.6%.16–20 This proportion was considered for unexposed patients, that is, those patients who received brand-name meropenem. The difference between the proportion among exposed and unexposed patients was considered; for the exposed patients who received generic medication, an increase in mortality of 68.5%21 was assumed. A statistical power of 80% and a confidence level of 95% were used, with an exposed/unexposed ratio of 1 and a loss of 10%. The calculated sample size was 140 patients in each arm, totaling 280 patients. Exposed and unexposed were matched for age (in 10-year ranges) and for type of infection. Sampling was performed by including non-probabilistic patients consecutively. Sample size was calculated in the program EPIDAT version 3.1. Because we were unable to complete the expected sample size, our power decreased 26.6%.
The study was conducted under the appropriate local and international ethics guidelines and approved by the institutional review board of the International Center for Training and Medical Research (CIDEIM, per its abbreviation in Spanish) and the participating institution. CIDEIM deemed that informed consent was not necessary for the study given that its design entailed minimal risk for the subjects.
Data collectionPatient data relevant to the study was retrieved from the hospital’s electronic medical record system and recorded on written data collection forms. Data collected included patient demographic information, hospital admission and discharge dates, ICU admission and discharge dates, clinical and laboratory data needed to calculate Sequential Organ Failure Assessment (SOFA) score, comorbidities, infection type, susceptibility profile of the infecting organism, antibiotic doses received during the infection episode, clinical and laboratory tests ordered between the infection episode and discharge, and survival status upon discharge.
Statistical analysisIn the descriptive statistical analysis, proportions were determined for qualitative variables, and mean or median calculated as determined by the distribution of quantitative variables. The odds ratio for mortality associated with the use of generic and brand-meropenem was computed using two-by-two contingency tables. Chi square or Fisher exact tests were used for comparisons of qualitative variables. For quantitative variables, parametric or non-parametric tests were used depending on whether distribution was normal or not. Survival analysis for 7- and 28-day survival both cohorts were compared using Log-rank test, adjusted for severity and adequate therapy. Both cohorts were matched for age and type of pathology. In the multivariate analysis, odds ratio was calculated using logistic regression with adjustment for the matching variables, and a conditional logistic regression was performed for death as an outcome. All possible models included paired variables, starting with those with a p-value <0.20 in the univariate analysis. In the multivariate analysis, p-value <0·05 was considered statistically significant.
Economic analysisThe cost of care for patients with infections that were selected for the study corresponded to the sum of the antibiotic consumption and length of stay until infection resolution. A partial economic assessment was carried out using micro-costing techniques as a way of determining the magnitude of the resources spent. Allocation of costs for each resource was based on a reference cost and adjusted by the inflation rate based on the Colombian manual document for costs.22 Incremental cost-effectiveness ratio (ICER), which is a measure of cost-effectiveness for a given intervention, was calculated using decision tree model. Survival was defined as the clinical outcome for effectiveness, while economic outcome was defined as the total infection cost per patient.
ResultsClinical impactA total of 1289 patients received meropenem during the study period, 318 patients received generic meropenem and 971 received brand-name meropenem. Of the 318 patients treated with generic meropenem, 68 (21.3%) met inclusion criteria for the study. Of the 971 patients treated with brand-name meropenem, 100 (10.3%) met these criteria. Thus, a total of 168 patients were included in the study. Demographic characteristics such as SOFA score, duration of antibiotic treatment, time of infusion and dosing of meropenem, as well as the microorganisms causing the infection were comparable among groups (Table 1). Regarding infection type, the most common was ventilator-associated pneumonia in the generic group (23% vs. 8%, p = 0.005) and abdominal infection in the brand-name group (13% vs. 34%, p = 0.004). On the other hand, the brand-name group had a higher prevalence of comorbidities such as type 2 diabetes (15% vs. 28%, p = 0.043) and cardiovascular disease (23% vs. 38%, p = 0.049). Also, history of hospitalization in the three months prior to hospital admission was more common in the brand-name group (53% vs. 72%, p = 0.011).
Univariate analysis.
| Variable | Generic meropenem group | Brand name meropenem group | p- value |
|---|---|---|---|
| n = 68 (41%) | n = 100 (59%) | ||
| Sex | 0.318 | ||
| Male | 42 (62%) | 54 (54%) | |
| Female | 26 (38%) | 46 (46%) | |
| Age (years) | 0.440 | ||
| 18–28 | 7 (10%) | 6 (6%) | |
| 29–39 | 7 (10%) | 16 (16%) | |
| 40–50 | 7 (10% | 11 (11%) | |
| 51–61 | 12 (18%) | 24 (24%) | |
| 62–72 | 14 (21%) | 19 (19%) | |
| >72 | 21 (31%) | 24 (24%) | |
| Age | 58.6 (SD ± 19.7) | 56.47(SD ± 17.45) | |
| Previous hospitalization | 36 (53%) | 72 (72%) | 0.011 |
| Comorbidities | |||
| Diabetes | 10 (15%) | 28 (28%) | 0.043 |
| Pulmonary disease | 8 (12%) | 16 (16%) | 0.441 |
| Immunosuppression | 3 (4%) | 7 (7%) | 0.742 |
| Neurologic disease | 7 (10%) | 2 (2%) | 0.032 |
| Renal disease | 6 (9%) | 4 (4%) | 0.319 |
| Liver disease | 1 (1%) | 1 (1%) | 1 |
| Cardiovascular disease | 16 (23%) | 38 (38%) | 0.049 |
| Cancer | 5 (7%) | 3 (3%) | 0.271 |
| Sequential Organ Failure Assessment (SOFA) score | 0.180 | ||
| 0–6 | 10 (40%) | 17 (52%) | |
| 7–9 | 7 (28%) | 11 (33%) | |
| 10–12 | 6 (24%) | 5 (15%) | |
| 13–14 | 2 (8%) | 0 (0%) | |
| SOFA | 7.72 (SD = 2.96) | 6.69 (SD = 2.59) | |
| Glasgow Coma Scale (GCS) score | 0.660 | ||
| Mild | 43 (65%) | 54 (69%) | |
| Moderate | 7 (11%) | 6 (8%) | |
| Severe | 16 (24%) | 18 (23%) | |
| Glasgow | 12.37 (SD ± 3.64) | 12.11 (SD ± 4.25) | |
| Invasive devices | |||
| Orotracheal tube | 62 (91%) | 85 (85%) | 0.235 |
| Central venous catheter | 60 (88%) | 83 (83%) | 0.349 |
| Foley catheter | 59 (87%) | 86 (86%) | 0.887 |
| Infection type | |||
| Bloodstream infection | 29 (43%) | 54 (54%) | 0.149 |
| Ventilator-associated pneumonia | 16 (23%) | 8 (8%) | 0.005 |
| Urinary tract infection | 9 (13%) | 10 (10%) | 0.516 |
| Skin and soft tissue infection | 3 (4%) | 4 (4%) | 1 |
| Meningitis | 2 (3%) | 1 (1%) | 0.565 |
| Intraabdominal infection | 8 (13%) | 33 (34%) | 0.004 |
| Dosing interval of meropenem | 0.290 | ||
| 6 hours | 1 (2%) | 1 (1%) | |
| 8 hours | 60 (88%) | 94 (94%) | |
| 12 hours | 7 (10%) | 3 (3%) | |
| 24 hours | 0 (0%) | 2 (2%) | |
| Prolonged meropenem infusion | 4 (10%) | 23 (24%) | 0.096 |
| Time between culture and initiation of meropenem treatment | 0.765 | ||
| Immediate | 26 (38.2%) | 19 (73%) | |
| 24–72 hours | 10 (14.7%) | 1 (4%) | |
| >72 hours | 12 (17.6%) | 1 (4%) | |
| already receiving | 1 (1.4%) | 0 | |
| Time between culture and initiation of meropenem | 18 (SD ± 39.01) | 43.7 (SD ± 53.13) | |
| Days of treatment with meropenem | |||
| <7 days | 1/49 (2%) | 0/14 (0%) | 0.778 |
| 7 days | 22/50 (44%) | 13/21 (62%) | 0.168 |
| Ceftriaxone | 16/51 (31%) | 9/23 (39%) | 0.514 |
| Ciprofloxacin | 18/53 (34%) | 6/22 (27%) | 0.572 |
| Multidrug resistant bacteria | 15 (22%) | 20 (20%) | 0.747 |
| Appropriate therapy | 66 (97%) | 99 (99%) | 0.351 |
| Infectious organism | |||
| Escherichia coli | 18 (27%) | 41 (43%) | |
| Klebsiella pneumoniae | 16 (24%) | 16 (17%) | |
| Enterobacter spp. | 7 (11%) | 5 (5%) | |
| Proteus spp. | 1 (1%) | 2 (2%) | |
| Pseudomonas aeruginosa | 8 (12%) | 18 (19%) | |
| Serratia marcescens | 3 (4%) | 6 (6%) | |
| Acinetobacter baumannii | 10 (15%) | 5 (5%) | |
| Morganella morganii | 3 (4%) | 3 (3%) | |
| Mortality | 42 (62%) | 41 (41%) | 0.008 |
We categorized the SOFA in lower and higher than 10, since the SOFA greater than 10 is associated with a mortality of more than 40%. On day 7 and 28 there were no differences in mortality by severity. In contrast, there was higher mortality at day 7 and 28, in patients using generic meropenem (Fig. 1).
Mortality was greater in the generic group (62% vs. 41%, p = 0.008); univariate analysis was performed to assess risk factors associated with mortality. Statistically significant variables were age greater than 73 (RR 4.98, 95% CI 1.31–18.96, p = 0.018), history of cardiovascular disease (RR 2.53, 95% CI 1.23–5.26, p = 0.02), presence of central venous catheter (RR 2.92, 95% CI 1.07–8.74, p = 0.02), ventilator-associated pneumonia (RR 6.43, 95% CI 1.99–26.9, p = 0.000), and treatment with generic meropenem (RR 2.32, 95% CI 1.18–4.59, p = 0.008), as shown in Table 2.
Bivariate analysis.
| Variable | Relative risk (95% CI /p-value) |
|---|---|
| Sex | 0.72 (0.37–1.38/p = 0.285) |
| Age | |
| 18–28 | (Reference group) |
| 29–39 | 0.47 (0.10–2.34/p = 0.359) |
| 40–50 | 1.80 (0.40–8.07/p = 0.443) |
| 51–61 | 2.51 (0.65–9.67/p = 0.180) |
| 62–72 | 2.39 (0.61–9.33/p = 0.210) |
| 73+ | 4.98 (1.31–18.96/p = 0.018) |
| Comorbidities | |
| Diabetes | 1.79 (0.88–4.03/p = 0.119) |
| Pulmonary disease | 1.25 (0.48–3.30/p = 0.614) |
| Immunosuppression | 1.03 (0.23–4.64/p = 0.969) |
| Neurologic disease | 0.81 (0.16–3.92/p = 0.760) |
| Renal disease | 1.02 (0.22–4.64/p = 0.969) |
| Cardiovascular disease | 2.53 (1.23–5.26/p = 0.006) |
| Solid Organ Tumor | 3.23 (0.55–33.48/p = 0.138) |
| Previous hospitalization | 1.19 (0.60–2.35/p = 0.597) |
| Sequential Organ Assessment Failure (SOFA) score | |
| 0–6 | (Reference group) |
| 7–9 | 1.96 (0.58–6.61/p = 0.276) |
| 10–12 | 0.71 (0.17–3.03/p = 0.648) |
| 13–14 | 1.25 (0.07–22.13/p = 0.879) |
| Glasgow Coma Scale (GCS) score | |
| Mild | (Reference group) |
| Moderate | 0.95 (0.30–3.03/p = 0.931) |
| Severe | 1.40 (0.64–3.08/p = 0.397) |
| Invasive devices | |
| Endotracheal tube | 2.75 (0.94–9.09/p = 0.041) |
| Central venous catheter | 2.92 (1.07–8.74/p = 0.020) |
| Foley catheter | 1.62 (0.61–4.52/p = 0.289) |
| Infection type | |
| Bloodstream infection | 0.56 (0.29–1.08/p = 0.064) |
| Vent.-assoc. pneumonia | 6.43 (1.99–26.90/p = 0.000) |
| Urinary tract | 1.88 (0.64–5.96/p = 0.203) |
| Skin and soft tissue | 1.38 (0.23–9.73/p = 0.676) |
| Meningitis | 2.1 (0.11–125.33/p = 0.539) |
| Intraabdominal | 0.45 (0.20–1.00/p = 0.034) |
| Time b/w culture and initiation of meropenem treatment | |
| Immediate | (Reference group) |
| <24 hours | 0.78 (0.31–1.96/p = 0.597) |
| 24–72 hours | 1.13 (0.43–3.02/p = 0.800) |
| 72+ hours | 0.96 (0.33–2.83/p = 0.941) |
| Obtained prior to ICU | 0.88 (0.40–1.96/p = 0.760) |
| Multidrug resistant bacteria | 1.28 (0.57–2.91/p = 0.516) |
| Use of generic meropenem | 2.32 (1.18–4.59/p = 0.008) |
| Infectious organism | |
| P. aeruginosa | 1.57 (0.61–4.20/p = 0.303) |
| Enterobacteria | 0.64 (0.24–1.63/p = 0.303) |
As both generic and brand-name meropenem groups were comparable by age and comorbidities in previous analyses, the multivariate analysis was adjusted for SOFA, GLASGOW and time of administration of meropenem. Exposure and cardiovascular disease were statistically significant.
Patients who received generics had 18-fold higher risk of dying compared with those who received brand-name (OR: 18.45 95%CI: 1.46–232) and patients with cardiovascular disease also had 18-fold higher risk of dying compared with those who did not have this comorbidity (OR: 18.1 95%CI: 1.26–262). In spite of the wide confidence intervals, due the small sample size, they did not include 1, with a good statistical significance (Table 3).
Multivariate analysis.
| Variable | Odds Ratio (95% CI /p-value) |
|---|---|
| Sequential organ failure assessment score | |
| (Group 1) | 1.07 (0.15–7.50/p = 0.943) |
| (Group 2) | 0.73 (0.08–6.62/p = 0.776) |
| (Group 3) | 0.64 (0.02–25.33/p = 0.812) |
| Glasgow score | |
| (Group 1) | 2.76 (0.20–37.98/p = 0.449) |
| (Group 2) | 31.33 (0.60–1639.36/p = 0.088) |
| Treatment with generic meropenem | 18.45 (1.45–232.32/p = 0.024) |
| Comorbidities | |
| Diabetes | 2.93 (0.40–21.38/p = 0.288) |
| Cardiovascular disease | 18.18 (1.25–262.63/p = 0.033) |
| Infection type | |
| Bloodstream | 3.40 (0.27–41.61/p = 0.338) |
| Vent.-assoc. pneumonia | 5.92 (0.16–208.88/p = 0.328) |
| Urinary tract | 2.49 (0.07–82.87/p = 0.609) |
| Intraabdominal | 4.54 (0.25–79.87/p = 0.301) |
| Time b/w culture and initiation of meropenem treatment | |
| (Group 1) | 0.64 (0.06–6.81/p = 0.717) |
| (Group 2) | 0.27 (0.02–2.97/p = 0.288) |
| (Group 3) | 0.05 (0.00–4.09/p = 0.189) |
| (Group 4) | 1.15 (0.09–14.71/p = 0.910) |
| Solid organ tumor | 3.11 (0.08–107.83/p = 0.530) |
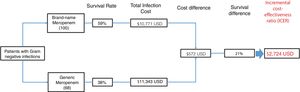
The total antimicrobial cost was lower in the brand-name group as compared to the generic group ($303 vs. $588). The total cost of ICU stay was also lower in the brand-name group ($8,896 vs. $7,705). However, the total cost of infection was not significantly different between groups (brand-name cost $10,771 vs. generic cost $11,343).
The ICER is a measure of cost-effectiveness for a given intervention, which in this case is the choice of antibiotic. The ICER was defined by the cost of obtaining one additional effectiveness unit (patient survival) was $2,724 USD per ICU stay when changing from brand name molecule to generic. The cost-effectiveness decision tree model is shown in Fig. 2.
DiscussionIn this study, we compared critically ill patients treated with generic versus brand-name meropenem. Results demonstrated greater mortality in the generic group and comparable total costs for both groups. History of cardiovascular disease was also found to be an independent risk factor for mortality. In contrast, the brand-name had a higher prevalence of comorbidities and history of hospitalization in the three months prior to hospital admission.
Meropenem is one of the most commonly-used antibiotics in critically ill patients due to its broad-spectrum bactericidal activity and its pharmacokinetic characteristics. The present study suggests clinical superiority of brand-name meropenem with similar costs in care. There are few clinical studies that compared clinical outcomes with treatment of generic versus brand-name antibiotics. Most studies have been conducted in vitro and animal models.23,24 Also, systematic reviews on the efficacy and quality of GAs have not been able to conclude their inferiority in relation to BNAs, maybe in part due to the heterogenous nature and different end points of the studies included, which precluded a definitive conclusion.25 Furthermore, it is difficult to have the possibility of evaluating brand versus generic meropenem in the same hospital, under the same conditions, like we were able to do in our study. For example in Thailand, a study comparing generic versus brand-name meropenem in hospitalized patients, of which 60% had documented microbiological data and some were infected with meropenem-resistant bacteria, did not find inferiority of either molecule.24 However, in our study the inclusion criteria was meropenem susceptible Gram-negative bacteria, eliminating the possibility that resistance could interfere with outcome ascertainment in the two groups. Another Colombian prospective cohort study that included 1015 patients with nosocomial infections showed in multivariate analysis a risk of death almost two-fold higher in patients treated with GAs compared to patients treated with BNAs (HR = 1.91; 95%CI = 1.43–2.55).21
Several studies led by Vesga et al. have compared GAs and BNAs using in vivo models. One of these studies using three different brands of generic vancomycin showed lower in vivo effectiveness compared to brand-name vancomycin despite similarities of chemical equivalence and potency. This difference was statistically significant and independent of route of administration or doses.7 Furthermore, Vesga et al. have shown difference in efficacy with other antibiotics, including meropenem, in which a pharmacologic equivalence did not translate into therapeutic equivalence in animal infection models.9 In this study, the authors proposed that differences in meropenem efficacy might be secondary to trisodium compounds that increase susceptibility in the face of hydrolysis within the organism. Finally, Vesga et al. also evaluated the impact of GA use on bacterial susceptibility profiles.8 An animal model was used to evaluate the resistance profile of Staphylococcus aureus in the face of successive cycles of GA and BNA. It was observed that generic vancomycin progressively selected subpopulations of resistant bacteria, confirming the effect of suboptimal bactericidal action of GA on microorganism susceptibility. In contrast to the Vesga et al. findings, some authors like Louie et al. and Hadwiger et al. have not been able to replicate these results.26,27 Irrespective of the contradictory findings with animal models, our study results show a statistically significant difference in the clinical outcomes.
Another key point is the therapeutic indications for GA. In the U.S., the Food and Drug Administration therapeutic indications for generic and brand-name meropenem are identical. However, the Colombian National Institute of Food and Drug Surveillance Agency (INVIMA in Spanish) has recommended only brand-name meropenem for the treatment of febrile neutropenia rather generic meropenem. Likewise, INVIMA recommends only brand-name vancomycin for the treatment of pneumonia, sepsis, or meningitis caused by penicillin-resistant Streptococcus pneumoniae; difference in the therapeutic indications between the GA versus the BNAs raises concerns whether GAs can be prescribed in all kinds of patients, especially when critically ill.
This study is different from other studies regarding the clinical outcomes between generic versus brand-name antibiotics. First, the selected study population was limited to critically ill patients. This population was selected because their conditions demand that antibiotics used have good effectiveness and safety due to severity of illness. Critical patients usually have alterations in the volume of distribution, tissue perfusion and renal functions among others, which may lead to significant challenges in achieving recommended pharmacokinetic and pharmacodynamic parameters for optimal treatment against bacterial infections.28 Second, only patients with documented Gram-negative infections susceptible to meropenem were included to maximize the likelihood of successful treatment with the selected antibiotics. To reduce confounding factors in the mortality analysis, patients with invasive fungal infections were excluded. Third, the economic analysis allowed the comparison of cost of hospitalization as well as the calculation of incremental cost of survival for each treatment used. Fourth, the groups of patients treated with generic and brand-name meropenem were comparable in terms of age and severity of infections. Furthermore, the group of patients treated with the brand-name molecule had a higher prevalence of cardiovascular disease, which was a significant risk factor for mortality.
Pharmacoeconomic analyses are important for an evaluation of costs and benefits of health interventions. In the present study, a cost-effectiveness model was created to compare costs when using each molecule. The net healthcare-associated costs were included in the assessment of each molecule, as well as the secondary costs of complications resulting from therapeutic failure and adverse reactions associated with the antibiotic. On the other hand, the cost-of-stay in the ICU was higher in the generic group due to the development of more complications and unfavorable clinical progression. At the end, there were no significant differences in the costs of treatment for each episode of infection between cohorts, which is the most cogent argument for using generics.
When the incremental cost effectiveness ratio (ICER) was calculated for the brand-name and generic molecules in our study, the ICER analysis showed that treatment with brand-name meropenem confers greater patient survival at a lower cost and is therefore is a better treatment option than its generic counterpart.
A limitation of this study is that only 67% of the anticipated sample size of 252 was achieved. This occurred primarily due to the inclusion and clinical follow-up criteria, which was strict and therefore led to the exclusion of many patients. Also, secondary to the smaller sample size achieved, some variables like SOFA or other non-cardiovascular comorbidities where not found to be associated with mortality in the multivariate analysis. While a smaller sample may alter the precision of the study, our results demonstrate a strong statistical association that is unlikely to be by chance. Even though the GA had a higher incidence of ventilator associated pneumoniae which may contribute in part to the higher mortality, the multivariate analysis did not show any difference associated with this type of infection. Also, as we explained before, we did not find any other factors that could explain the higher mortality with GA like changes in the antibiotic prescription or infection control practices.
In conclusion, the use of brand-name meropenem for the treatment of Gram-negative infections susceptible to carbapenems in the ICU is a more cost-effective option than generic meropenem. Institutions should follow clinical and microbiologic outcomes of patients treated with antibiotics, which in turn may allow for early detection of therapeutic failure. Ultimately, this approach can provide data that will help drug producers improve the development of antibiotics.
Financial supportThis study was funded by the International Center for Medical Training and Research (CIDEIM in Spanish). This study did not receive any funding from drug manufacturers or any other sources.
Conflicts of interestThe authors declare no conflicts of interest.
ThanksWe are grateful for the assistance we received from Obed David Suárez Anaya, Carmen Elisa Llanos Uribe, Luz Stella Salazar, and Camila Marin Peralta.
Author informationKaren Ordóñez designed the study, collected and interpreted data, revised the manuscript, and approves of the final version. Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Visualization, Writing original draft, writing review and editing. Address: Carrera 18#12-75 Torre 1 Consultorio 1205, Postal Code 660003, Pereira, Colombia.
Max M. Feinstein collected and interpreted data, drafted the manuscript, and approves of the final version. Address: 2058 E. 115th St., Cleveland, Ohio, Postal Code 44106, USA. Data curation, formal analysis, writing original draft, writing review and editing.
Sergio Reyes collected and interpreted data, revised the manuscript, and approves of the final version. Data curation, formal analysis, investigation, supervision, validation, writing original draft. Address: Av. La Maria #19-225, Postal Code 760031, Cali, Valle del Cauca, Colombia.
Cristhian Hernández-Gómez designed the study, interpreted the data, revised the manuscript, and approves of the final version. Conceptualization, Formal analysis, investigation, methodology, software, supervision, validation, writing original draft, project administration writing review and editing. Address: Cra 9 # 131 A- 02, Lab de Investigacion 2 Piso, Postal Code 110121, Bogotá, Colombia.
Christian Pallares designed the study, interpreted the data, revised the manuscript, and approves of the final version. Conceptualization, formal analysis, methodology, software, validation, writing original draft, project administration, writing review and editing. Address: Av. La Maria #19-225, Postal Code 760031, Cali, Valle del Cauca, Colombia.
María V. Villegas designed the study, revised the manuscript, and approves of the final version. Conceptualization, funding acquisition, project administration, resources, supervision, validation, visualization, writing-review and editing. Address: Cra 9 # 131 A- 02, Lab de Investigacion 2 Piso, Postal Code 110121, Bogotá, Colombia.
All authors agree to be accountable for all aspects of the work and ensure the accuracy and integrity of the study.