The prison system in Paraná, Brazil, is experiencing serious problems related to the increasing number of prisoners. Control of hepatitis C virus (HCV) has become more intense because the incarcerated population is considered a high-risk group for contagious diseases due to the favorable conditions found in prisons for the spread of these morbidities. The objective of this study was to identify features associated with hepatitis C infection among male prisoners in correctional institutions of Paraná state, Brazil.
MethodsThis was a case-control study (27 cases and 54 controls) of men incarcerated in 11 penitentiaries in Paraná, Brazil. Information was obtained through a questionnaire in a cross-sectional epidemiological survey on HCV infection during the period from May 2015 to December 2016. Eligible men were recruited after testing positive for anti-HCV antibodies. Cases and controls were selected based on serological results of enzyme-linked immunosorbent assays and were matched by age, location of the penitentiary, and time in prison. Logistic regression analysis was used to identify risk factors for HCV seropositivity.
ResultsThe main significant independent risk factor for the acquisition of HCV infection was the use of injectable drugs (OR = 4.00; 95%CI:1.41–11.35; p < 0.001).
ConclusionsThis study provides evidence that HCV infection is associated with drug use by this population. This information is pivotal for tailoring prevention programs and guiding specific socioeducational measures that aim to reduce or prevent HCV transmission within the prison setting.
Infection with type C viral hepatitis (HCV) is common among the prison population. It is also thought that incarceration itself may increase the risk of infection.1,2 This increased risk can be attributed to several causes, some associated with the prisons’ structural and logistic problems and others with common problems or behaviors acquired during seclusion.2,3 Worldwide, it is estimated that approximately 15.1% of the 10.2 million incarcerated individuals are living with HCV.4 This rate is lower than the prevalence reported among prisoners in international studies, including studies in Italy (22.4%),5 Indonesia (34.1%)6 and Azerbaijan (38.2%).7
A wide variation in anti-HCV prevalence in the incarcerated population has been reported, ranging from 1% in a study carried out in Colatina, Brazil,8 to 47.4% in a study carried out in Australia.9 In a systematic review by Magri et al., hepatitis C prevalence ranged from 1% to 41% in Brazil.1 This variation is associated with differences in geographical prison location, age, previous prison sentences, intravenous drug use, and even the quality of public health services available to the population, as well as with the different habits and high-risk behaviors in different geographical regions.10–13
Contamination by HCV in prisons has been linked to trauma-induced blood exposure, tattooing, intravenous drug use, and sexual activity.1,2,9,14–19 Confinement plus additional factors (sharing of material used for drug use, tattoos, piercings, and razor blades in addition to inadequate sterilization or reuse of medical or dental instruments) and deficiencies in health care also increase the vulnerability of prisoners to HIV/AIDS and other sexually transmitted infections.1,15,20–23
Although there is some understanding of the scenarios that lead to the transmission of infectious diseases in prisons, epidemiological studies are still scarce for this population, especially in Brazil. Recently, a systematic review of studies from 1989 to 2014 conducted by Magri et al. on the prevalence of hepatitis C in prisoners in Brazil confirmed that none of the reviewed studies had been conducted in the state of Paraná, Brazil.1 This study aimed to analyze the factors associated with hepatitis C infection among inmates of the Paraná prison system.
Materials and methodsThis study was preceded by a cross-sectional survey carried out from May 2015 to December 2016 in 11 penitentiaries in Paraná state entitled “Prevalence of HIV and Hepatitis B and C in the prison population in the state of Paraná.” Based on information from the Penitentiary Department of Paraná (DEPEN, PR) at the time of the study, there were approximately 19,000 prisoners in 23 closed male prisons. Eleven of the 23 high-security closed prisons in six cities of the state (Francisco Beltrão, Londrina, Curitiba, São José dos Pinhais, Pinhais, and Piraquara) were included (Fig. 1). Our criteria for selecting the participating cities considered both the size of the cities as well as the presence of penitentiaries in closed regime. Three cities – all located in the state of Paraná – were then selected to take part in this study (Francisco Beltrão, a small-sized city, with a population of up to 100 thousand inhabitants; Londrina, a relatively large city, but with less than one million inhabitants; and Curitiba, the capital city with more than one million inhabitants).24 In the closed-regime systems, inmates remain under daily supervision within the prison limits.
Geographic locations of the penitentiaries included in the study in Paraná, Brazil.
PEL, State Penitentiary of Londrina; CRESLON, Social Reintegration Center of Londrina; PEP, State Penitentiary of Piraquara; PCE, Central State Penitentiary; CCJP, House of Custody of São José dos Pinhais; CCC, House of Custody of Curitiba; CCP, House of Custody of Piraquara; CMP, Criminal Medical Complex; PFB, State Penitentiary of Francisco Beltrão; RM, Metropolitan Region of Curitiba.
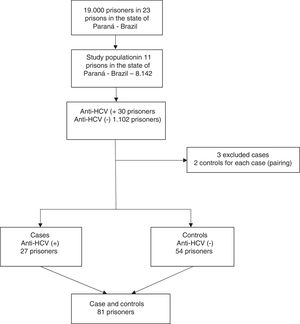
The sample size was calculated based on the expected prevalence of 50% HCV infection with 1% variation, a power of 80%, and 3% alpha error. The study population included 8,142 inmates, and the study sample size was 954 inmates. Approximately 25% more individuals (total: 1192 inmates) were added to account for anticipated loss due to participation refusal. Proportional stratified sampling was performed using each prison as a randomization unit. A total of 1132 prisoners were interviewed, with 60 losses or refusals (5%). On the day of data collection, the prisoners were sorted numerically in ascending order from the lists provided by the prison, and a list of random numbers was generated using the software Microsoft Excel® 2007. To be included, patients had to be (a) aged 18 years or older; (b) in custody, (c) able to consent for themselves, (d) able to be interviewed only by a researcher (without risk markers), and (e) able to understand spoken Portuguese. This study was carried out with the approval of the research Ethics Committee of the State University of West Paraná under approval number 810.574.
All eligible participants provided voluntary informed written consent prior to participation in the study and no compensation was provided. The treatment offered to the individuals who did not take part in the study was the same as that given to participants. Prisoners identified as HCV-positive received medical assistance. To investigate the prevalence of anti-HCV antibodies, we used a 3rd generation chemiluminescent enzyme test (Architect® anti-HCV assay — Germany) in serum samples on a i4000 instrument (Architect System, United States of America [USA]). Test results were analyzed according to the manufacturer’s instructions.
Based on the results of the above cross-sectional survey, we planned this case-control study during the period of March through May 2018 to investigate the risk factors associated anti-HCV seropositivity among men incarcerated in the closed regime of the penitentiary system of Paraná, Brazil. In the first study, 30 prisoners anti-HCV reactive were identified. Out of these, 27 cases from eight penitentiaries of Paraná were matched for same penitentiary, time of imprisonment within five years, and age within three years with 54 control subjects. Three cases were excluded because it was not possible to find matched controls for them. A case was defined as any inmate with reactive serology for HCV. Controls were selected from among prisoners with serologic markers for non-reactive HCV. A flowchart of the recruitment strategy is shown in Fig. 2.
After cases and controls selection, the following information was obtained: race/color, marital status, schooling, occupation when incarcerated, number of times in prison, conviction time, STD history, knowledge about hepatitis B and C transmission routes, blood transfusion history, tattoo and piercing history, illicit drug use, injectable drug use, condom use, alcoholic beverage use, sexual orientation, homosexual relationship history, and intimate visit history. Anonymous identification numbers were used to cross-reference the information contained in the questionnaires.
All processing and analysis steps were performed with the Statistical Package for Social Science, version 24.0 (SPSS Inc., Chicago, IL, USA) program. Absolute (n) and relative (%) frequencies are used to describe the sample features. Initial comparisons between groups were performed by factorial ANOVA (groups vs penitentiaries). Comparisons between cases and controls for categorical variables were performed using chi-square tests with continuity correction. Variables that presented p < 0.20 in univariate analysis were included into a binary logistic regression model to identify independent risk factors for HCV infection (p < 0.05).
ResultsHCV seroprevalence identified in the survey “Prevalence of HIV and Hepatitis B and C in the prison population of the Paraná State Penitentiaries” was 2.7% (95%CI 1.9–3.8). Cases (n = 27) and controls (n = 54), selected concomitantly, were not significantly different in terms of penitentiary, age, and time of imprisonment (Table 1).
Description of the paired variables of the cases (27) and controls (54) according to the penal unit, average age, and prison time in the state prisons of Paraná.
| Penitentiary | Sample N | Average age | Average prison time | |||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |
| PFB | 01 | 02 | 32a | 31,00 ± 1.14 | 9a | 7 ± 0.00 |
| PEL I | 02 | 04 | 45.50 ± 2.12 | 45.25 ± 2.63 | 4.50 ± 2.12 | 5.00 ± 3.74 |
| PEL II | 04 | 08 | 45.25 ± 11.53 | 44.63 ± 10.24 | 3.50 ± 2.08 | 3.75 ± 2.60 |
| PEC | 07 | 14 | 42.29 ± 8.14 | 42.21 ± 7.89 | 4.71 ± 2.69 | 5.36 ± 3.93 |
| PEP II | 05 | 10 | 32.60 ± 9.81 | 33.10 ± 8.60 | 1.80 ± 1.92 | 2.00 ± 2.40 |
| CCC | 03 | 06 | 39.33 ± 3.78 | 38.17 ± 3.43 | 1.67 ± 2.08 | 2.00 ± 1.26 |
| CCP | 03 | 06 | 30.67 ± 9.02 | 30.67 ± 8.07 | 0.33 ± 0.57 | 0.16 ± 0.40 |
| CMP | 02 | 04 | 39.50 ± 0.71 | 44.50 ± 11.03 | 0.50 ± 0.71 | 0.75 ± 1.50 |
| Total | 27 | 54 | 38.96 ± 9.10 | 39.13 ± 9.26 | 14.25 ± 14.06 | 15.11 ± 12.63 |
Effects of ANOVA information for case vs control: age (F = 0.017; p = 0.896) and prision time (F = 0.001; p = 0.999).
Table 2 shows the general characteristics of the two groups. The mean age was 39 years, and positive serology was predominantly found among individuals over 30 years of age. Although the differences were not statistically significant, the cases had a higher prevalence of unemployment, had been in prison more often, reported greater knowledge about hepatitis, and had higher prevalence of blood transfusions, illicit drug use, and homosexual relationships than the controls. However, the only variable independently associated with anti-HCV seropositiveness after multivariate analysis was injectable drug use (OR: 4.0 95%CI 1.41–11.35).
Sociodemographic characteristics, risk factors, prison variables and HCV infection among male prisoners of the penitentiaries of the state of Paraná, Brazil (n = 81).
| Cases (n = 27) | Control (n = 54) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Skin color | ||||||||
| White | 13 | 48.1 | 29 | 53.7 | 0.814 | |||
| Other | 14 | 51.9 | 25 | 46.3 | ||||
| Marital state | ||||||||
| Married/cohabitating | 11 | 40.7 | 28 | 51.9 | 0.479 | |||
| Single/divorced | 16 | 59.3 | 26 | 48.1 | ||||
| Education | ||||||||
| Incomplete elementary school | 14 | 51.9 | 27 | 50.0 | 0.875 | |||
| Complete elementary education | 13 | 48.1 | 27 | 50.0 | ||||
| Were employed | ||||||||
| Yes | 9 | 33.3 | 26 | 48.1 | 0.303 | |||
| No | 18 | 66.7 | 28 | 51.9 | ||||
| Number of times in the prison system | ||||||||
| Up to 2 times | 5 | 18.5 | 19 | 35.2 | 0.197 | |||
| More than 2 times | 22 | 81.5 | 35 | 64.5 | ||||
| Conviction time | ||||||||
| Up to 12 years | 15 | 55.6 | 26 | 48.1 | 0.694 | |||
| More than 12 years | 12 | 44.4 | 28 | 51.9 | ||||
| DST history | ||||||||
| Yes | 11 | 40.7 | 26 | 48.1 | 0.693 | |||
| No | 16 | 59.3 | 28 | 51.9 | ||||
| Knowledge about hepatitis | ||||||||
| Yes | 14 | 51.9 | 20 | 37.0 | 0.301 | |||
| No | 13 | 48.1 | 34 | 63.0 | ||||
| Blood transfusion | ||||||||
| Yes | 7 | 25.9 | 8 | 14.8 | 0.363 | |||
| No | 20 | 74.1 | 46 | 85.2 | ||||
| Tattooing | ||||||||
| Yes | 19 | 70.4 | 32 | 59.3 | 0.464 | |||
| No | 8 | 29.6 | 22 | 40.7 | ||||
| Piercing | ||||||||
| Yes | 5 | 18.5 | 12 | 22.2 | 0.923 | |||
| No | 22 | 81.5 | 42 | 77.8 | ||||
| Illicit drugs use | ||||||||
| Yes | 24 | 88.9 | 36 | 66.7 | 0.060 | |||
| No | 3 | 11.1 | 18 | 33.3 | ||||
| Injecting drug use | ||||||||
| Yes | 12 | 44.4 | 9 | 16.7 | 0.016 | |||
| No | 15 | 55.6 | 45 | 83.3 | ||||
| Condoms use | ||||||||
| Yes | 14 | 51.9 | 32 | 59.3 | 0.418 | |||
| No | 4 | 14.8 | 9 | 16.7 | ||||
| Do not know | 9 | 33.3 | 13 | 24.1 | ||||
| Alcohol consumption | ||||||||
| Yes | 24 | 88.9 | 49 | 90.7 | 0.794 | |||
| No | 3 | 11.1 | 5 | 9.3 | ||||
| Sexual orientation | ||||||||
| Heterosexual | 25 | 92.6 | 47 | 87.0 | 0.708 | |||
| Other | 2 | 7.4 | 7 | 13.0 | ||||
| Homosexual relationship | ||||||||
| Yes | 5 | 18.5 | 3 | 5.6 | 0.148 | |||
| No | 22 | 81.5 | 51 | 94.4 | ||||
| Intimate visit | ||||||||
| Yes | 7 | 25.9 | 19 | 35.2 | 0.556 | |||
| No | 20 | 74.1 | 35 | 64.8 | ||||
STD, sexually transmitted diseases; Fr, frequency.
Four variables with a p < 0.20 (Table 2) were included in the logistic regression model to identify independent risk factors for anti-HCV reagent (Table 3). Of these, two variables persisted significantly associated: illicit drug use and injectable drug use. As the two presented high collinearity (those who used illicit drugs were the same individuals who used injecting drugs), construction of a multiple regression model was not possible. However, given the narrow confidence interval of the OR in the initial analysis, injecting drug use is the main risk factor for hepatitis C infection in prisoners.
Independent hepatitis C predictors of anti-HCV seropositiveness in men arrested in the penitentiary system in Paraná by multivariate logistic regression analysis.
| ORbr (IC 95%) | p | |
|---|---|---|
| Number of times in the prison svstem | ||
| Up to 2 times | 1 | |
| More than 2 times | 2.39 (0.78–7.32) | 0.128 |
| Illicit drugs use | ||
| Yes | 4.00 (1.06–15.08) | 0.041 |
| No | 1 | |
| Injecting drug usea | ||
| Yes | 4.00 (1.41–11.35) | <0.001 |
| No | 1 | |
| Homosexual relation | ||
| Yes | 3.86 (0.85–17.60) | 0.081 |
| Nn | 1 | |
There have been few studies in Brazilian male prisons assessing risk factors for hepatitis C infection.1 Most of these studies have aimed just to determine the prevalence of HCV seromarkers.12 Therefore, among the contributions of this study, we highlight the case-control design with representation of prisoners in 11 closed-regime male penitentiaries in the state of Paraná. It is worth mentioning that this is the first study in the state that shows a clear view of the prevalence of HCV and the risk factors related to exposure among prisoners.
The prison population is considered to be at high risk for acquiring infectious diseases1,25 because prisoners are confined and present risky behavioral factors such as low educational level,25 irregular and infrequent use of condoms, multiple sexual partners, sex under the influence of alcohol and drugs,26–28 and irregular use of preventive measures.1 These risk factors and behaviors can precede arrest and frequently continue during incarceration.18,29
Prisoners with a history of previous incarcerations have had a greater opportunity to acquire HCV infection than those who have not been previously incarcerated.1,18 Several studies have revealed that previous history of incarceration is related to frequent exposure to high-risk activities such as unsafe lifestyles, risky sexual behaviors, exposure to sharps for tattooing, and sharing of needles, syringes and other paraphernalia for illicit drug use.1,12,18,25
Recently, Stone et al. published a systematic review and meta-analysis of 41 studies on this subject. It has been identified that both recent and past incarceration contribute to an increased risk of HCV acquisition ranging from 21% to 62%.30 In the present study, previous incarceration was significantly associated with HCV infection in univariate analysis. However, this factor was not considered an independent predictor of viral infection, suggesting that there must be other factors that confound the relationship between these two variables.29
Studies have identified that homosexual relationship offer a greater chance of HCV infection than heterosexual relationship; in our study, in the final model, this association was not confirmed.28–32 It is believed that this association may be related to risk behaviors rather than to homosexuality itself, according to Reekie et al.33
In this study, illicit drug use was associated with HCV exposure. These findings have also been identified in other studies worldwide and in Brazil.18,25,29,32 Despite the significance of illicit drug use in univariate analysis, in the final model only injecting drug use was associated with positive serology for HCV, since those who used illicit drugs were the same individuals who used injecting drugs (collinearity). In our study, the odds of contracting hepatitis C were four-fold higher for men who used injecting drugs (OR = 4.00; 95%CI: 1.41–11.35) than for prisoners in the same prison system who did not inject drugs. Injecting drug-using prisoners and males in a meta-analysis study were 24 times more likely to be HCV-positive than non-injecting drug users.26
Worldwide, there is a continuing epidemic of HCV infection among young white adults who inject drugs, are aged less than 30 years, and with a history of previous or simultaneous opiate consumption.33,34 HCV infection prevalence is 10–15 times higher among people who inject drugs, with higher rates among new injectors. A quarter of injecting drug users will be infected within two years after starting. In addition, HCV is approximately 10 times more infectious than HIV, and injecting drug use increases the risk by 3–10% compared to 0.3% for HIV contamination. Furthermore, the virus remains infective in liquid and syringes and on inanimate surfaces for weeks.35–37
The relationship between illicit drug use, HCV infection and imprisonment is very close, as injecting drug users (IDUs) are the individuals with the highest rates of incarceration due to involvement in illicit activities, and they get involved criminal activities to finance their addictions.38 A recent meta-analysis of six published studies on the incidence of HCV in prisoners reported that the risk of HCV infection was eight times higher in injecting drug-using prisoners than in injecting drug-using non-prisoners.30
In a cohort of 735 Australian prisoners with a history of injecting drug use that were followed for more than 14 years, 55.1% of prisoners were found to have some history of HCV, and 47.4% of those tested in the prison were seropositive for HCV. In this study, drug injection in prison was found to be strongly associated with HCV seropositivity by Snow et al.9 Roux et al. also reported injecting drug use in 99% of cases in their survey of sociodemographic and behavioral characteristics of participants tested for HCV infection in a prison population in southwestern France.39
In Latin America and the Caribbean, 11.3% of prisoners coinfected with HIV and hepatitis C use injecting drugs, and in Brazil, the prevalence is approximately 9.2%.40 In Brazilian studies, there are reports of syringe sharing for drug use in 32.5% of prisoners. The use of illicit drugs is common in prison environments, and even when the use of only inhaled illicit drugs has been reported, high frequency of use ends up being a bridge to injectable drug use.41 Pereira et al., in their study on populations from 26 Brazilian states and the Federal District, showed in a multivariate model that use of injectable drugs (OR = 6.65; 95%CI: 2.47–17.91) and of inhaled drugs (OR = 2.59; 95%CI: 1.34–5.81) were predictive factors for HCV infection.41
The epidemiological impact of various risk factors for acquiring HCV infection has been investigated in a number of studies on prison populations, and injecting drugs has been found to be the main risk factor associated with HCV infection in prison populations.9,10,30,37 Although such risk behavior is strictly prohibited in prisons around the world, almost half of illicit drug users continue to use such substances after arrest. In addition, the difficulty in obtaining sterile injecting equipment in prison results in widespread sharing of infected equipment and increased risk of HCV transmission.12
It should be clarified that the use of injecting drugs is also a risk factor outside the prison environment. In developed countries, most new HCV infections are reported in injecting drug users. The most recent surveys of active IDUs in the United States indicate that approximately one third of young users (ages 18–30) are infected with HCV.42
The seroprevalence of HCV observed in this study was 2.7%, which is higher than that in the range of 0.7% and 1.38% reported among people aged 10–69 years.41,43,44 In this study, HCV seroprevalence was slightly higher than that found in a study by Puga et al.18 among men in penitentiaries in the state of Mato Grosso do Sul (2.4%), but lower from the rates found in studies on prisoners in the states of São Paulo (8.7%) and Minas Gerais (6.34%).24,45 The prevalence of HCV infection among prisoners in Paraná was also lower than that found in studies in Australia (29%), England (24.2%), Italy (22.4%), Ghana (18.7%), Iran (7.4%), and Lebanon (3.4%).46–48
The presence of HCV can be manifested as an acute infection, with development of symptoms in 20–30% of cases, or as a chronic infection that can result in liver fibrosis, cirrhosis, or hepatocellular carcinoma in 70–80% of cases.33,34 The possibility of infection without apparent symptoms makes it difficult to diagnose infection early and, consequently, postpones diagnosis and treatment of chronic patients. This situation of delayed diagnosis is frequent within the prison system since the infrastructure and health services in the system to care for the prisoner are poor, when existent.48 There is also an absence of educational campaigns and guidance for the practice of safe sex, sexual transmission among men who have sex with other men (HSH) is higher in the prison environment than in the outside environment, reaching rates of 8,6%. In relation to the HIV-positive prisioners, a report from Israel showed a rate of 6.5% among HSH who had anal sex before imprisonment.49
Educational campaigns and guidelines for the practice of safe sex among the prison population would be useful for reducing the prevalence rates of HCV and other sexually transmissible diseases. Lack of healthy behavior is related to the low effectiveness of the medical teams in prison environments, which hinders medical care, since prisons are environments characterized by conditions of insecurity and fear.50
This study has limitations because some behavioral risks may have been underreported by the participants due to the fact that vulnerability can lead to discrimination and stigmatization. Such underreporting could have contributed to underestimation of the potential risk factors associated with HCV exposure.
In conclusion, we observed injectable drug use to be the main risk factor for hepatitis C infection in men incarcerated in the penitentiary system of Paraná state, Brazil. The increased risk was associated with a high frequency of risky behavior before and during incarceration, especially for injecting drug users. These results suggest an urgent need for effective prevention programming, education, prison strategies, and programs adapted to local contexts as well as new direct-acting antiviral drugs, treatment strategies, and therapies.
Serological testing for screening and monitoring of IDUs and treatment of drug abuse and addiction among prisoners in need in the prison system would make it possible to evaluate the success of harm reduction strategies (needle/syringe sharing programs for injecting drugs and tattoos). Finally, studies using phylogenetic analysis will also enhance understanding of the routes of HCV transmission in this population.
Conflicts of interestThe authors declare no conflicts of interest.
This research was funded by the Ministry of Health (Brazilian Government) and Western Paraná State University (UNIOESTE) under agreement 797322/2013.