Human papillomavirus (HPV) infection is common in sexually active women and viral persistence may cause intraepithelial lesions and eventually progress to cervical cancer (CC). The present study aimed to investigate epidemiological factors related to HPV infection and to evaluate viral persistence and CC precursor lesions frequencies in women from a city in the countryside of South Brazil. Three hundred women were recruited from a primary public health care clinic. The patients were interviewed and underwent sampling with cervical brushes for HPV-DNA detection/typing by a PCR-based assay and cytological analysis by Pap smear test. HPV was detected in 47 (15.7%) women. HPV infection was significantly associated with young age (<30 years) and low socio-economic status. Seventeen (5.7%) women presented cytological abnormalities, three of them with precursor CC intraepithelial lesions. A subgroup of 79 women had been previously analyzed and thirteen (16.4%) were persistently infected, two with precursor CC intraepithelial lesions and high-risk HPV types infection (both of them without cervical abnormalities in the first exam). In conclusion, HPV infection was associated with young age (<30 years) and low family income; viral persistence was low (16.4%) but related to CC precursor lesions; and HPV-DNA high risk types detection would help to screen CC in the population.
Human papillomavirus (HPV) is one of the most common causes of sexually transmitted diseases in the worldwide. It has the ability to infect epithelial and may resist asymptomatic or cause a variety of diseases, including cancer.1 HPV infection is usually transient and most people eliminate the virus from the body with the effective action of the immune system after 5.1–15.4 months.2 However, HPV persistence can cause benign lesions, known as warts (in different parts of the body), or low/high grade intra-epithelial lesions (LSIL/HSIL) that can progress to cancer, mainly in the uterine cervix.3 Further, HPV persistence and consequently cervical cancer (CC) depends on other factors, such as age, high parity, smoking, long-term use of contraceptives, sexual behavior and co-infection with other sexually transmitted infectious agents.4
HPV prevalence ranges from 13.7% to 54.3% according to the studied population and geographic area in Brazil reviewed by.5 However, main epidemiological studies have been performed in the capitals and metropolitan cities. These studies demonstrated that HPV infection was associated with multiple sexual partners6; young age, more lifetime sex partners and abnormal vaginal flora7 and non-stable sexual partners.8 HPV persistence also presented frequencies as different as 19.2% in Porto Alegre (Rio Grande do Sul state) and 59.6% in Ouro Preto (Minas Gerais state) in two studies performed in primary public health care clinics from Brazil.9,10
This study aimed to investigate epidemiological aspects associated with HPV infection and to evaluate HPV persistence in women from the city of Cruz Alta and surrounding small localities. This countryside region is located in the North of the Rio Grande do Sul (the southern most state in Brazil) and it is more than 300km away from the respective capital city (Porto Alegre).
MethodologyStudy population and sample collectionA cross-sectional study was conducted with 300 women who accepted to participate in the study while attending for CC screening in a primary public health care clinic (Center for Women and Children) in the city of Cruz Alta (Rio Grande do Sul State, Brazil) from January 2012 to April 2013. Epidemiological informations (socio-demographic, behavioral, and clinical) were obtained from a standardized individual questionnaire that was administered by a trained interviewer in a private room. The research project was approved by the Research Ethics Committee of the University of Cruz Alta (Protocol No. 078.0.417-09).
After each participant gave informed consent, cervical samples were collected from all participants for HPV-DNA testing and cytological analysis. Clinical samples were collected by scraping the ectocervix and endocervix of each patient with an endocervical brush, smeared on a glass slide (that was fixed immediately with polyethylene glycol for cytological examination) and after stored in a buffer solution (EDTA pH 8.0 0.01M, SDS 0.03M), and stored at −20°C until analysis.
Women also enrolled in a previous report11 or with two visits in this study (minimum interval of twelve months) were identified to evaluate HPV persistence. A total of 79 (26.3%) women attended these criteria (57 evaluated in the previous study and 22 analyzed twice in this study) and composed a subgroup to investigate HPV persistence.
The clinical management of the patients was in accordance with the “Brazilian classification for cervical reports and recommended procedures: recommendations for health professionals”.12 This protocol do not establish HPV testing in the routine screening, so results of HPV types were not used in the management of the patients.
HPV-DNA detection and typingHPV-DNA testing was performed by nested polymerase chain reaction (nested-PCR) and restriction fragment length polymorphism (RFLP) as previously described.13 Samples presenting insufficient DNA for HPV typing were classified as inconclusive. Results were interpreted by two independent analysts and HPV types were classified into high-risk (HR) and low-risk (LR).14
Cytological analysisThe cytological analysis was performed by conventional Pap smear test evaluated by two independent cytologists (conflicting results were submitted to a third evaluation). Cell abnormalities were classified according to the Bethesda System 2001.15 Basically, it classifies the modified cells into nine categories (five to squamous and four to glandular cells): (1) squamous cell carcinoma, (2) high-grade squamous intraepithelial lesion (HSIL), (3) low-grade squamous intraepithelial lesion (LSIL), (4) atypical squamous cells of undetermined significance (ASC-US), (5) atypical squamous cells that cannot exclude HSIL (ASC-H), (6) adenocarcinoma, (7) endocervical adenocarcinoma in situ, (8) atypical glandular cells (AG), and (9) atypical glandular cells not otherwise specified (AG-NOS). Normal cells were defined as negative for intraepithelial lesion and malignancy (NILM).
HPV persistenceWomen with two evaluations were classified into four categories according to the HPV infection status: (1) persistent infection: HPV-DNA positive in both assessments; (2) conversion: HPV-DNA negative in the first visit and HPV-DNA positive in the follow-up; (3) elimination (clearance): HPV-DNA positive only in the first evaluation; (4) without HPV infection: HPV-DNA negative in both visits.
Statistical analysisData analysis was conducted using the SPSS version 17.0 software (SPSS Inc., USA). Association between HPV infection status and other variables was determined with the chi-square test. Multivariate models were conducted using a modified Poisson regression16 to test the independent associations of HPV infection with socio-demographic, behavioral and clinical characteristics. Associations that presented values of p between 0.05 and 0.15 in bivariate analysis were regarded as having borderline significance and were included in the modeling of confounding factors. All p values presented are two-tailed and the values of p<0.05 were considered statistically significant.
ResultsOf the 300 women evaluated in the study, HPV was detected in 47 (15.7%). Of these positive samples, 26 (55.3%) had single and 10 (21.3%) multiple HPV type infections (it was not possible to determine the type in the remaining 11 positive samples). A total of 23 viral types were identified; including 15 (65.2%) HR and 8 (34.8%) LR. Types HR most frequents were 16 (n=6), 31 (n=4), 45 (n=4) and 56 (n=3). Other HR types were 18, 35, 39, 52, 53, 58, 59, 68, 70, 73 and 82. Types LR most frequents were 6 and 81 (n=3; each). Other LR types were 11, 42, 44, 55, 64 and 84.
HPV positive women were younger (33.9±17.0 years) than the HPV negative ones (41.8±14.7 years; p<0.001). Other socio-demographic characteristics significantly associated to HPV infection were: to have a total household income lower than one Brazilian minimum monthly wage (p<0.001), not to be married (p=0.014) and to have children (p=0.016) (Table 1).
Analysis of socio-demographic characteristics in women according to HPV status.
| Variables | Overall (n=300) | Without HPV (n=253) | With HPV (n=47) | p-Valuea |
|---|---|---|---|---|
| Age (years) | 0.003 | |||
| ≤19 | 28 (9.3) | 19 (7.5) | 9 (19.1) | |
| 20–29 | 56 (18.7) | 41 (16.2) | 15 (31.9) | |
| 30–39 | 59 (19.7) | 50 (19.8) | 9 (19.1) | |
| 40–49 | 60 (20.0) | 57 (22.5) | 3 (6.4) | |
| 50–59 | 59 (19.7) | 53 (20.9) | 6 (12.8) | |
| ≥60 | 38 (12.7) | 33 (13.0) | 5 (10.6) | |
| Schoolingb | 0.247 | |||
| Elementary or lower education | 178 (60.8) | 153 (62.2) | 25 (53.2) | |
| Medium or higher education | 115 (39.2) | 93 (37.8) | 22 (46.8) | |
| Total household income (in Brazilian minimum monthly wage) | <0.001 | |||
| ≤1 | 126 (42.0) | 93 (36.8) | 33 (70.2) | |
| 2–3 | 162 (54.0) | 151 (59.7) | 11 (23.4) | |
| >3 | 12 (4.0) | 9 (3.6) | 3 (6.4) | |
| Marital statusb | 0.014 | |||
| Married or stable union | 56 (56.6) | 54 (60.7) | 2 (20.0) | |
| Single/divorced/widowed | 43 (43.4) | 35 (39.3) | 8 (80.0) | |
| Childrenb | 0.016 | |||
| Yes | 232 (78.1) | 203 (80.6) | 29 (64.4) | |
| No | 65 (21.9) | 49 (19.4) | 16 (35.6) | |
Data are reported as number with percent in parentheses.
Consistent condom use was significantly associated to the HPV infection (p<0.001). Other behavioral characteristics (age at first intercourse, number of lifetime sexual partners, smoking and contraceptive oral use) did not present association with HPV detection (Table 2).
Analysis of behavioral and clinical characteristics in women according to HPV status.
| Variables | Overall (n=300) | Without HPV (n=253) | With HPV (n=47) | p-Value* |
|---|---|---|---|---|
| Age at first intercourse | 0.248 | |||
| <18 | 175 (58.3) | 144 (56.9) | 31 (66.0) | |
| ≥18 | 125 (41.7) | 109 (43.1) | 16 (34.0) | |
| Lifetime sex partners | 0.147 | |||
| 1 | 144 (48.0) | 126 (49.8) | 18 (38.3) | |
| ≥2 | 156 (52.0) | 127 (50.2) | 29 (61.7) | |
| Smoking | 0.674 | |||
| Yes | 39 (13.0) | 32 (12.6) | 7 (14.9) | |
| No | 261 (87.0) | 221 (87.4) | 40 (85.1) | |
| Contraceptive oral use | 0.259 | |||
| Yes | 106 (35.3) | 86 (34.0) | 20 (42.6) | |
| No | 194 (64.7) | 167 (66.0) | 27 (57.4) | |
| Condom use | <0.001 | |||
| Yes | 92 (30.7) | 67 (26.5) | 25 (53.2) | |
| No | 208 (69.3) | 186 (73.5) | 22 (46.8) | |
| History of STD | 0.275 | |||
| Yes | 48 (16.0) | 43 (17.0) | 5 (10.6) | |
| No | 252 (84.0) | 210 (83.0) | 42 (89.4) | |
| Concurrent vaginal infection | 0.977 | |||
| Yes | 57 (19.0) | 48 (19.0) | 9 (19.1) | |
| No | 243 (81.0) | 205 (81.0) | 38 (80.9) | |
| Last Pap test | 0.031 | |||
| First time | 37 (12.3) | 27 (10.7) | 10 (21.3) | |
| ≤1 year | 150 (50.0) | 124 (49.0) | 26 (55.3) | |
| ≥2 years | 113 (37.7) | 102 (40.3) | 11 (23.4) | |
| Pap test | <0.001 | |||
| Normal | 279 (94.3) | 243 (96.1) | 36 (76.6) | |
| Abnormal | 17 (5.6) | 6 (2.3) | 11 (23.4) | |
| Unsatisfactory sample | 4 (1.4) | 4 (1.6) | 0 (0.0) | |
Data are reported as number with percent in parentheses.
Regarding clinical aspects, HPV infection was associated to the occurrence of cell abnormalities in the Pap smear test (p<0.001). Further, HPV was more frequent in women that underwent a Pap test in the last twelve months than in the other ones (p=0.031). History of sexually transmitted diseases and concurrent vaginal infection were not associated to HPV infection.
All the above epidemiological variables with a statistical significance in the chi-square test were submitted to the multivariate analysis (excepting marital status that presented a high number of missed data and the expected occurrence of cell abnormalities in the Pap smear test in HPV positive women). HPV infection was associated to the total household income lower than one Brazilian minimum monthly wage (p=0.001), young age (<30 years old; p=0.028) and use of condom in all relations (p=0.023) (Table 3).
Multivariate analysis of risk factors to HPV infection in women in South Brazil (n=300).
| Variable | PR adjusted | CI (95%) | p-Value |
|---|---|---|---|
| Age | 0.028 | ||
| ≥30 years | 1 | – | |
| <30 years | 2.00 | 1.08–3.72 | |
| Total household income | 0.001 | ||
| >1 Brazilian minimum monthly wage | 1 | – | |
| ≤1 Brazilian minimum monthly wage | 2.74 | 1.55–4.86 | |
| Have children | 0.438 | ||
| Yes | 1 | – | |
| No | 1.28 | 0.68–2.42 | |
| Lifetime sexual partner | 0.262 | ||
| 1 partner | 1 | – | |
| ≥2 partner | 1.36 | 0.80–2.31 | |
| Use of condom in all sexual relations | 0.023 | ||
| No | 1 | – | |
| Yes | 1.87 | 1.09–3.22 | |
| First Pap test | 0.761 | ||
| No | 1 | – | |
| Yes | 0.91 | 0.48–1.71 | |
In the cytological analysis, most women did not present cell abnormalities (n=279; 93%), 243 (87.1%) without HPV and 36 (12.9%) with HPV. Seventeen (5.7%) women presented cells alterations, fourteen (4.7%) of them only with atypical cells, one (0.3%) with LSIL and two (0.7%) with HSIL. The patient with LSIL was infected by HR type 45, while the women with HSIL were infected with the HR type 16 and LR type 16 plus 6 (mixed infection). Four samples presented unsatisfactory cytology results caused by contamination with blood cells, pus and mucus in over 75% of the smear (Table 4).
Cytological diagnosis according to the presence of HPV infection.
| Cytological diagnosis | Without HPV (N=253) | With HPV (N=47) | All women (N=300) |
|---|---|---|---|
| NILM | 243 (96.0) | 36 (76.6)a | 279 (93.0) |
| ASC-US | 4 (1.6) | 6 (12.8)b | 10 (3.3) |
| ASC-H | 1 (0.4) | 1 (2.1)c | 2 (0.7) |
| ASC-H+AG | 0 (0.0) | 1 (2.1) | 1 (0.3) |
| LSIL | 0 (0.0) | 1 (2.1)d | 1 (0.3) |
| HSIL | 0 (0.0) | 2 (4.3)e | 2 (0.7) |
| AG-NOS | 1 (0.4) | 0 (0.0) | 1 (0.3) |
| Unsatisfactory sample | 4 (1.6) | 0 (0.0) | 4 (1.4) |
Data are reported as number with percent in parentheses. NILM: negative for intraepithelial lesion and malignancy; ASC-US: atypical squamous cells of undetermined significance; ASC-H: atypical squamous cells, cannot exclude high-grade lesion; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; AG: atypical glandular cells; AG-NOS: atypical glandular cells not otherwise specified.
Includes sixteen cases of infection single by HPV types high risk: 16 (3 cases), 31 and 53 (2 cases each), 35, 45, 58, 70, 73 and 82 (1 case each) and three cases of infection multiple by HPV types 52 and 68; 31 and 45; 55 and 56; also includes seven cases of infection single by only HPV types low-risk: 6, 11, 42, 44, 64, and 84 (1 case each) and one case infection multiple by HPV types 6 and 81.
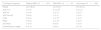
In the subgroup of 79 women with two visits, 46 women (58.2%) showed no HPV infection in both assessments. Almost all of them (n=44; 95.7%) had normal Pap smear test on both visits, but two (4.3%) presented atypical results (one ASC-US and another ASC-US plus AG-NOS, both in the second visit). Eighteen (22.8%) women eliminated HPV infection, presenting normal cells in both Pap smear tests. Only two (2.6%) women showed conversion to an HPV-DNA positive status, but without cytological alterations. The remaining thirteen (16.4%) women had persistent HPV infection, most of them with an HR type in the baseline test (n=11; 84.6%). All these women presented normal Pap smear in the first visit and ten (76.9%) had this same result in the second exam, while three (23.1%) presented cells abnormalities. One of these women had AG associated to a persistent infection with HR type 45, another presented LSIL associated to mixed infections of HR types in both visits (18 plus 33 in the first test and 16 plus 18 in the second one) and the last one presented HSIL with a mixed infection of HR type 52 and 73 in the first visit and single infection with HR type 52 in the follow-up (Table 5).
Course of HPV infection and classification of viral type in women from South Brazil.
| Course of infection | Alla (N=79) | HPV baseline (N=33) | |
|---|---|---|---|
| High-risk (N=19) | Only Low or indeterminate risk (N=14) | ||
| Persistence | 13 (16.4) | 9 (47.3) | 4 (28.5) |
| Clearance | 18 (22.8) | 8 (42.2) | 10 (71.5) |
| Conversion | 2 (2.6) | 2 (10.5) | 0 (0.0) |
| No HPV | 46 (58.2) | – | – |
Data are reported as number with percent in parentheses.
The present work is a cross-sectional study with women attended in a public health service in a city from the countryside from South Brazil. In this region, HPV infection was associated with four socio-demographical (young age, marital status, parity and total household income), one behavioral (consistent condom use) and two clinical (cell alterations in the Pap test performed in the present study and previous Pap testing in a period of one year) aspects.
Young age is a classical independent factor associated to HPV infection and it is well reported in the scientific literature.8,17,18 It is related to the more intense sexual activity in this age (that favors the infection) as well as to an anatomical characteristic of the young women (cervical ectopy) that exposes the columnar epithelium in the ectocervix, making it more vulnerable to pathogen infections.19,20
It was also observed a significant higher proportion of single, divorced or widowed than married women with HPV infection in the bivariate analysis. However this variable was not included in the multivariate analysis because the limited data available (n=99, less than one third of the total sample population). Other studies have also found this association, probably because unmarried women have a higher risk behavior than these ones living with a stable partner.21–23
Furthermore, it was found a significantly higher proportion of HPV infected women with children than those without children in the bivariate analysis. However this association was not significant in the multivariate analysis. In the literature, some studies found a positive association10,24,25 while others reported no significant relation between parity and HPV infection.6,26
Interestingly, total household income of less than one minimum Brazilian monthly wage (that means low socio-economy status) was strongly and significantly associated to HPV infection in the bivariate and even in the multivariate analyses wage (p=0.001). Similar findings were reported in other countries27,28 and also in the Northeastern from Brazil.29 Another study also reported that HPV was more frequent in public health services than in private clinics, highlighting the role of the socioeconomic status in HPV infection.30 As a global context, poverty or insufficient income is a social determinant for increased vulnerability of women and can either influence the adoption of preventive measures against sexually transmitted diseases or reduce the access to information and health services.31 This reinforces the need for the development of prevention and control programs in low-income populations.
Several sexual behaviors have been associated to HPV infection in other Brazilian reports.6,8,32,33 In the present study, HPV infection was surprisingly associated with consistent condom use. Condom offers good protection against infections, but HPV can be transmitted by contact with genital areas not covered by this preservative.34 Previous studies have reported a protective effect of condoms in preventing HPV infection, but without statistical significance.35,36 In addition, misuse or problems with condoms (breakage, slippage or incomplete use with delayed placement, early removal and even reuse) are factors that may offer less protection, as reported previously.37 Condom use may also have been more reported than effectively done in the practice, considering that the use is socially desirable.34 Finally, women with new and/or multiple sexual partners use condoms more frequently than those who only have one regular partner and could be related to a higher risk behavior as previously reported.34,38
On the other hand, it was not observed association between HPV infection and the number of lifetime sexual partners, age at first intercourse and history of sexually transmitted disease and other concomitant genital infection demonstrated in previous studies.6,32 Another study conducted in the South Brazil did not find association of these characteristics and HPV infection.39
In the cytological analysis, a low percentage of women without HPV infection (4.1%) had abnormal Pap smear while a higher frequency of women with HPV infection (23.4%) presented atypical cells and/or LSIL/HSIL as expected. Regardless of the degree of the lesion, all of them presented HR HPV types. In fact, normal cytology predominates among women without HPV infection, as shown in previous studies in Brazil7,26,40 and even in other countries.17,41
HPV persistence in this population was 16.4%. Further, persistent HR HPV types infection was associated with abnormal Pap smear in three patients (one AG, one HSIL and one LSIL). The frequency of AG is low, ranging from 0.05% to 2.1%, but this cytological abnormality has clinical significance (several cases progress to CC).42 On contrast, LSIL and HSIL are relatively common, accounting for 31% and 9.7% (respectively), among women with cytological abnormal cervical cells.43 In this situation, HPV-DNA test would be useful to monitor the occurrence of cervical lesions in women.43–45
Pap smear test is an effective method adopted for the CC screening and should be performed once a year in each woman aged 25–64.43 This procedure significantly reduced the incidence of cervical cancer in Brazil in the last five decades. However several new CC patients have been detected each year and this disease is still one of the main causes of death in women.43 There is consistent evidence on the performance of HPV-DNA testing combined with cytological analysis to detect cervical lesions with more accuracy. Some countries in Europe and North America have adopted HPV-DNA testing in clinical practice. The latest CC screening guidelines of several international health institutions recommended the adoption of cytology and HPV-DNA testing for any woman.46,47 However, HPV-DNA testing in combination with cytology is not routinely performed in the public clinics in Brazil. The introduction of HPV-DNA testing could help in the screening of precursor lesions and in the effective CC control.43,46,48,49
Screening programs for cervical lesions in women have been implemented in the last decades in Brazil. Such efforts aim to detect cancer precursor lesions and to provide early treatment for the patients, reducing the incidence of uterine cervical cancer in women. The present study was conducted in one of this public primary health care services located in a medium-size city from the South of Brazil. Although women of only one city were included in the study, the majority of them is of low socioeconomic status, representing women attended by the health public services in other cities and regions of the country. In this sense, the data reported here contribute to a better understanding of the HPV epidemiology and will be helpful to define public health policies in Brazil.
In conclusion, the results of this study indicate that age lower than 30 years and low family socioeconomic status are associated to HPV infection in women from the countryside of South Brazil. Further, it was detected a prevalence of HPV infection of 15.7% and a relatively low HPV persistence (16.4%), strongly related to CC precursor lesions. HPV-DNA high risk types detection would help to screen CC in the population.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the staff of the Center for Women's Health in Cruz Alta/RS and patients for their collaboration. We also thank the technicians of the Universidade de Cruz Alta (Cytopathology Laboratory), Universidade Luterana do Brasil (Molecular Diagnostics Laboratory) who performed technical support and Simbios Biotecnologia for the partial financial support. This work was also supported by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS; Grant 1265-2551/13-4).