Papiliotrema laurentii is one of several non-neoformans cryptococci that have rarely been associated with human infection, since it was previously considered saprophyte and thought to be non-pathogenic to humans. Nevertheless, increasing number of reports of human infection have emerged in recent years, mostly in oncologic patients.
AimTo report a case of a female patient with pyloric obstructive cancer with a catheter-related Papiliotrema laurentii blood stream infection and systematically review the available evidence on P. laurentii infection in humans.
MethodsRetrieval of studies was based on Medical Subject Headings and Health Sciences Descriptors, which were combined using Boolean operators. Searches were run on the electronic databases Scopus, Web of Science, MEDLINE (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), Cochrane Library for Systematic Reviews and Opengray.eu. There was no language or date of publication restrictions. The reference lists of the studies retrieved were searched manually.
ResultsThe search strategy retrieved 1703 references. In the final analysis, 31 references were included, with the description of 35 cases. Every patient but one had a previous co-morbidity - 48.4 % of patients had a neoplasm. Amphotericin B was the most used treatment and only a single case of resistance to it was reported. Most patients were cured of the infection.
ConclusionP. laurentii infection in humans is usually associated to neoplasia and multiple co-morbidities, and amphotericin B seems to be a reliable agent for treatment.
The advancement of Medicine brought new medications, therapeutics, invasive diagnostic methods and surgical approaches have in different pathologies. However, new obstacles emerge to defy our scientific knowledge. Rare pathogens until then unknown take advantage of health fragility in humans to cause infections with alarming proportions.
Cryptococcus spp. other than C. neoformans and C. gattii were previously considered to be saprophytes and non-pathogenic to humans; however, opportunistic infections associated with rare Cryptococcus spp., such as Cryptococcus laurentii and Cryptococcus albidus, have increased over the past four decades.1Cryptococcus laurentii belongs to the phylum basidiomycota of the fungi and is an encapsulated saprobic yeast and can be widely isolated from various types of environments.2 It is widely distributed throughout the world, including the Caribbean, Antarctic and the Himalayas and can be acquired from air, water, wood, soil, pigeon excrements as well as various foods, such as cheese, fruit, pork products, bean, and wine.3 Since 2015, the species name Cryptococcus laurentii was replaced by Papiliotrema laurentii. This nomenclature was based on phylogenetic analyses based on the sequencing of seven genes and regions such as ITS rRNA gene, the D1/D2 domains of the large subunit (LSU or 26S) rRNA gene, the small subunit (SSU or 18S) rRNA gene, two subunits of RNA polymerase II (RPB1 and RPB2), translation elongation factor 1-α (TEF1) andcytochrome b (CYTB).
With increasing immunosuppression due to antineoplastic therapy, organ transplantation, catheter insertion, dialysis and other invasive diagnostic and therapeutic procedures, systemic fungal infections are observed more frequently.4 Non-neoformans cryptococci have been reported to cause infection in many organs. The bloodstream and central nervous system are the most common sites of non-neoformans cryptococcal infection.5 Due to the rarity of cases involving P. laurentii, a standard treatment has not yet been established. Commonly, amphotericin B with flucytosine is recommended.6
The aim of this paper was to report a case of a female patient with pyloric obstructive cancer with catheter-related Papiliotrema laurentii bloodstream infection and systematically review the available evidence on P. laurentii infection in humans.
Case reportA 68-year old female patient, previously diagnosed with type 2 diabetes, arterial hypertension, non-alcoholic fatty liver disease, with a previous history of breast cancer which was treated with radical mastectomy, radiotherapy and chemotherapy a decade ago, sought care due to weight loss (20 % of total body mass - over 40 pounds), incoercible vomiting, weakness, hypoglicemia and upper abdominal pain.
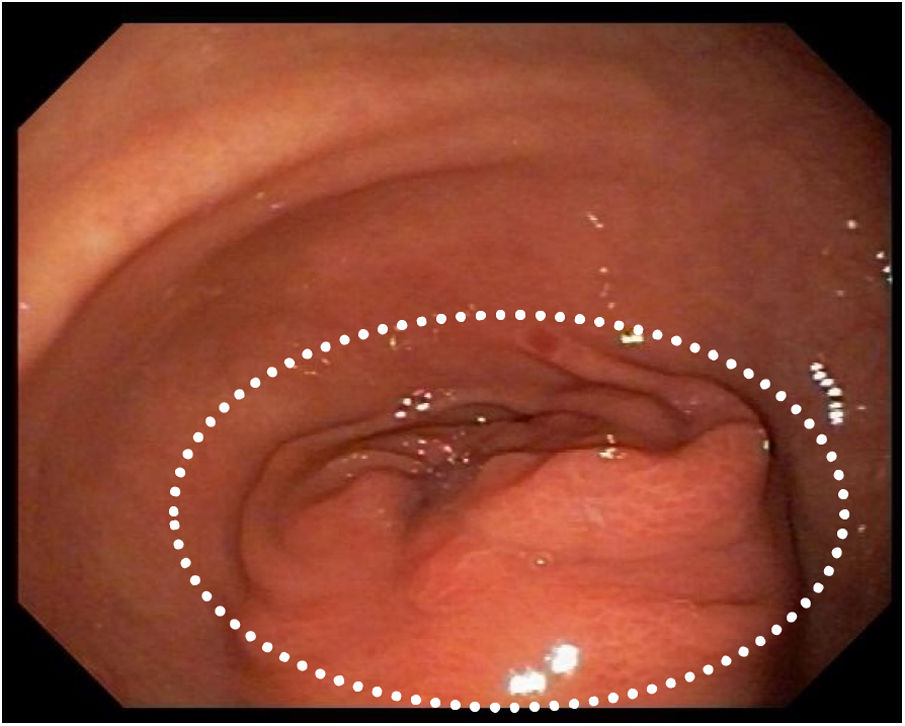
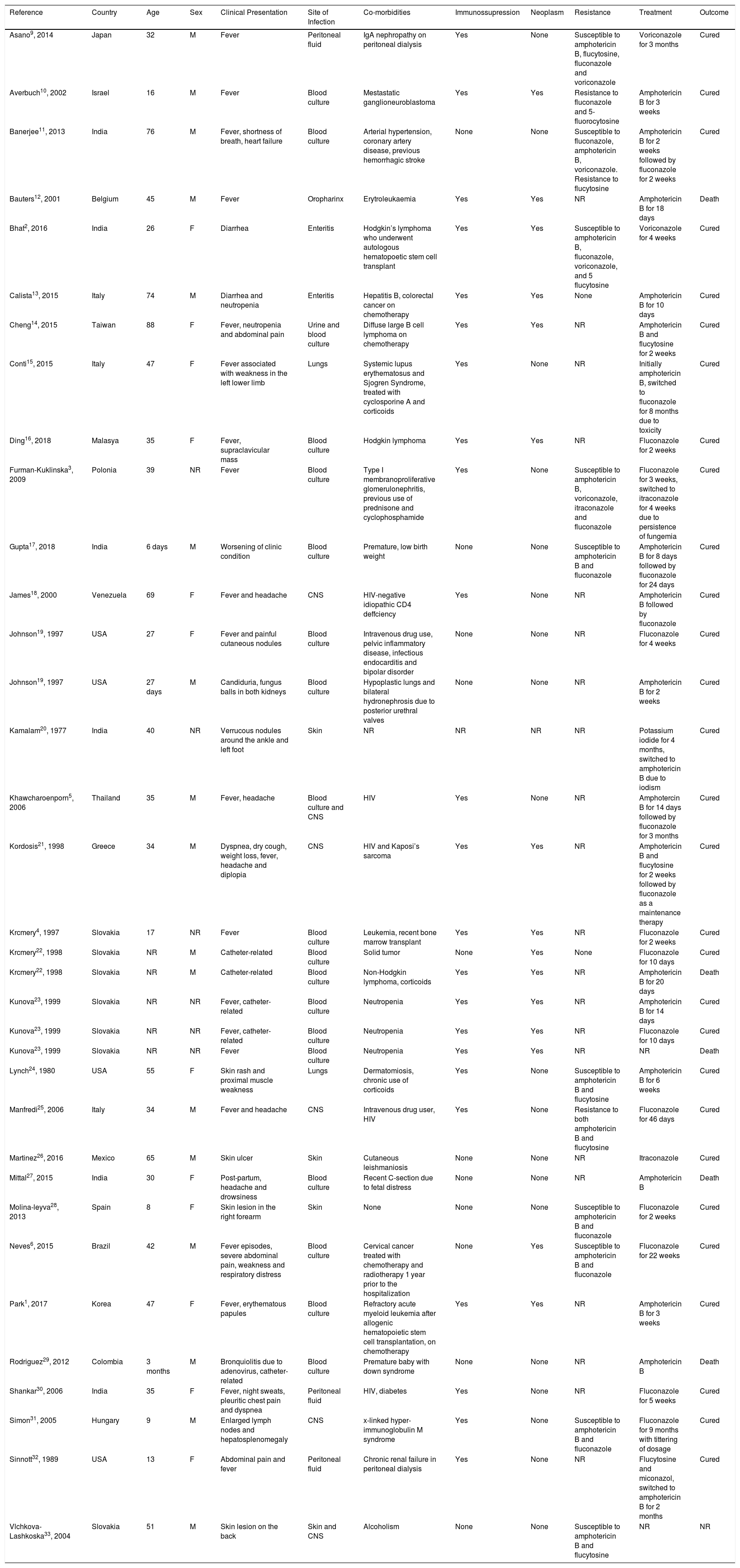
She was admitted to the hospital for investigation. CAT-scan showed gastric distension. Upper digestive endoscopy showed submucosal obstructive pyloric malignancy (Fig. 1), but superficial biopsies came back negative for cancer. Colonoscopy was incomplete due to inadequate colonic preparation - the patient vomited manitol. Magnetic resonance showed a pyloric-duodenal mass, suggestive of submucosal pyloric cancer. A two-week parenteral nutrition (PN) was initiated with the purpose of improving nutrition prior to surgery. Patient gained five pounds while on PN. In the 12th day of PN, the patient began to present fever. Blood cultures were drawn and ampicillin-sulbactam was initiated with little response.
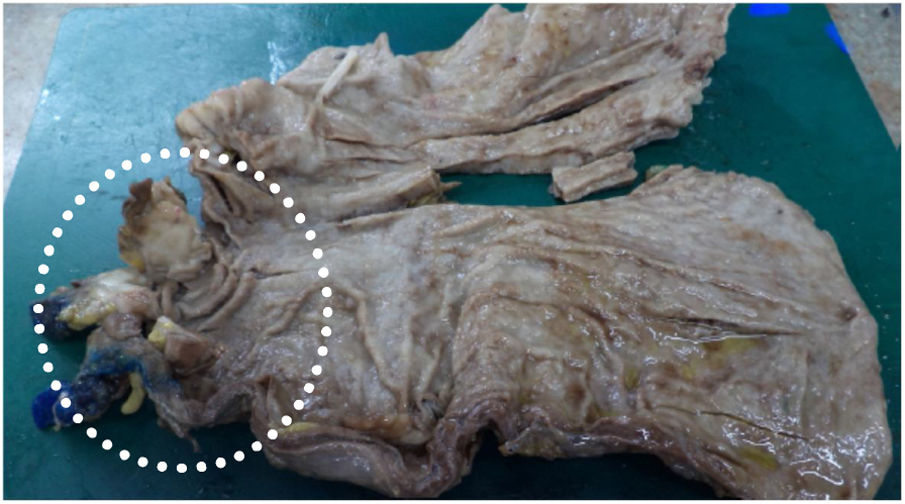
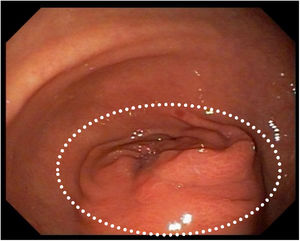
Five days thereafter, the patient presented with bacteremia. Catheter and peripheral cultures drawn during fever came back positive for Papiliotrema laurentii, with antifungigram pending. Identification of the isolate was performed on the Vitek 2 ( YST card - BioMérieux, Marcy l'Etoile, France) automated identification system, which reported P. laurentii. The concern regarding misdiagnosis by Vitek systems was minimized since the P. laurentii culture had been sent to two different laboratories and regrown for the antifungigram, which confirmed the first and second P. laurentii diagnosis with the same resistance profile. The isolate sent to the second lab was again identified by the same Vitek 2 (BioMérieux, Marcy l'Etoile, France) yeast identification card with testing staff blinded to previous Vitek 2 and antifungigram results. Xiao et al. cited that compared to the gold standard (identification of ITS- internal transcribed spacer), Vitek 2 can correctly identify 81.0 % of P. laurentii isolates.7 Fluconazole, piperacillin-tazobactam and vancomycin replaced ampicillin-sulbactam and the site of the catheter was switched. In five days, fever subdued and after 5-day negative control blood cultures, partial gastrectomy with Y-en-Roux gastroenteric anastomosis was performed (Fig. 2). With an adequate evolution, patient began to eat orally and PN was reduced gradually.
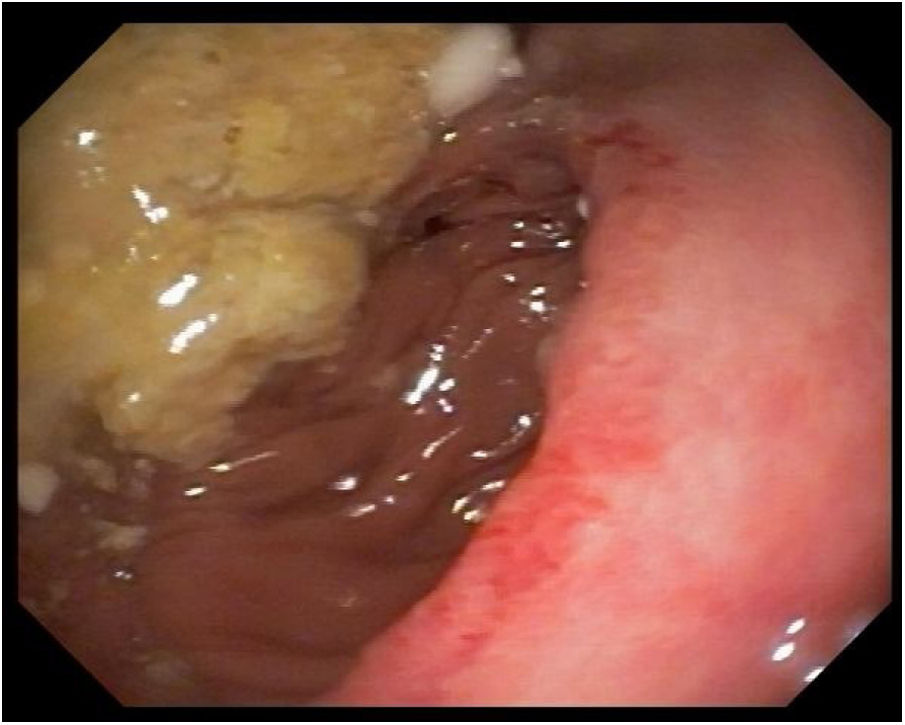
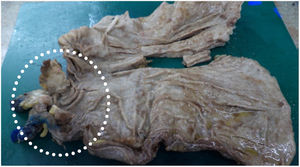
Five days after surgery, fever was again noted and catheter and peripheral cultures were positive for P. laurentii and Candida parapsilosis. The first was susceptible to flucytosine (intermediary), fluconazole, amphotericin B, voriconazole, and resistant to micafungin and caspofungin. The latter was susceptible to flucytosine, fluconazole, amphotericin B, voriconazole, micafungin and caspofungin. Transesophageal echocardiogram was negative for infective endocarditis. Vancomycin and piperacillin-tazobactam were already finished and patient was on monotherapy with fluconazole 800 mg once a day. Catheter site was switched again and amphotericin B 50 mg once a day was associated, with resolution of fever. After 14 days of combined therapy and negative peripheral cultures, patient was discharged with a dosage of 800 mg oral fluconazole daily. A post-operative control CAT-scan and an upper digestive endoscopy were performed, showing no signs of recurrence of the neoplasm (Fig. 3).
Materials and methodsThis study was carried out in accordance with the recommendations contained in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA-P) guidelines.8 Our systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO), maintained by York University, on 14 January 2019 [registration No. CRD42019122125 (www.crd.york.ac.uk/prospero/)].
Data sourcesStudies were retrieved using the term “Cryptococcus laurentii”. Searches were run on the electronic databases Scopus, Web of Science, Medline (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), Cochrane Library for Systematic Reviews and Opengray.eu. There was no language or date of publication restrictions. The reference lists of the retrieved studies were submitted to manual search. Databases were searched January 2019.
Inclusion criteria and outcomesCase report or case series studies were eligible for selection. If there was more than one study published using the same case, the most recent study was selected for analysis. Studies published only as abstracts were included, as long as the data available made data collection possible. The outcome measured was cure of the infection or death.
Study selection and data extractionAn initial screening of titles and abstracts was the first stage to select potentially relevant papers. The second step was the analysis of the full-length papers. Two independent reviewers extracted data using a standardized data extraction form after assessing and reaching consensus on eligible studies. The same reviewers separately assessed each study and extracted data about the characteristics of the subjects and the outcomes measured. A third reviewer was responsible for clearing divergences in study selection and data extraction.
Statistical analysisData was summarized using descriptive analysis – frequency and means.
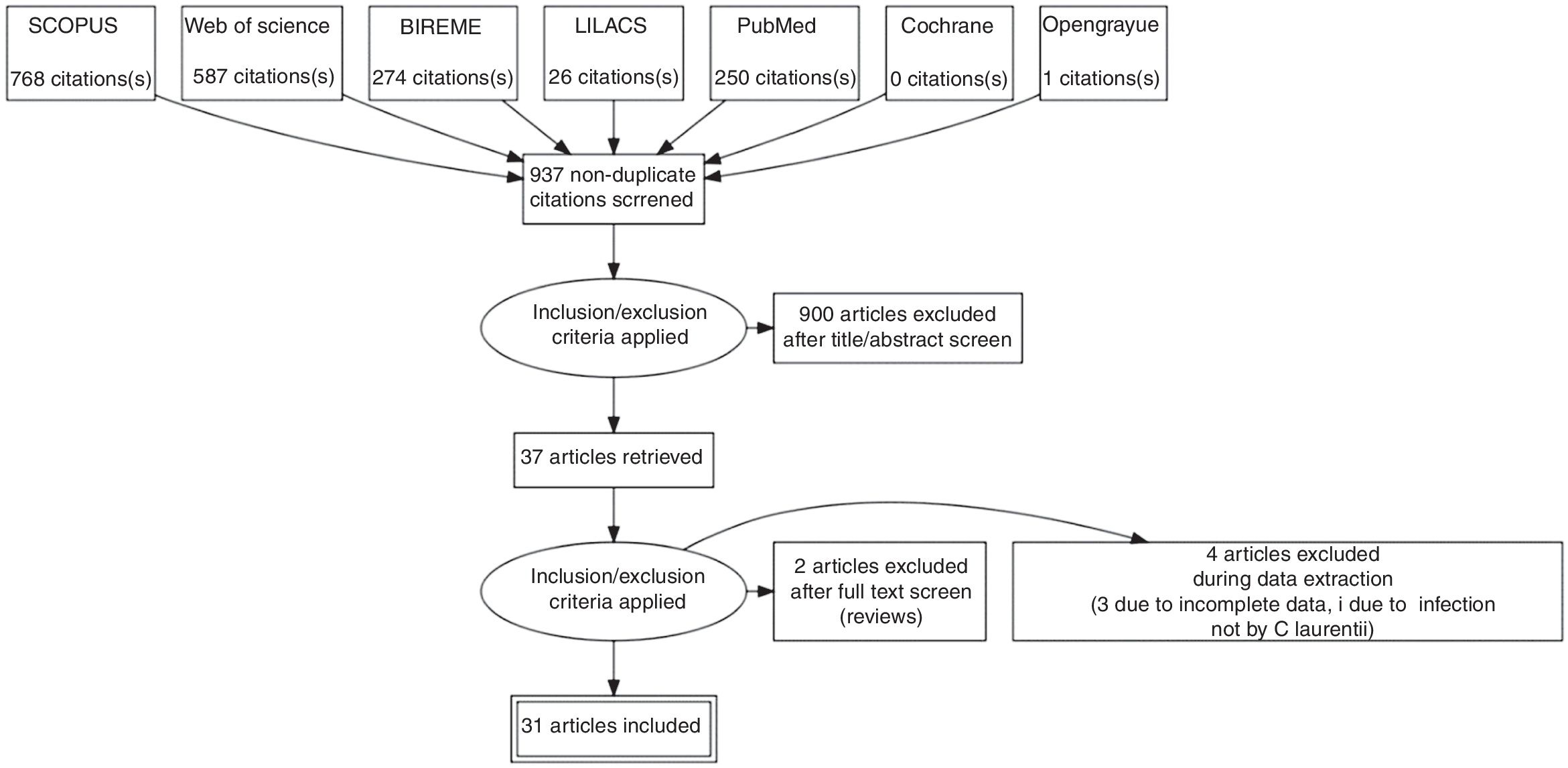
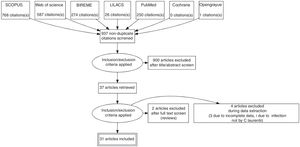
ResultsSystematic reviewThe search strategy retrieved 1703 references, 767 references were excluded because they were duplicates. After analyzing titles and abstracts, 900 references were excluded. Full texts were retrieved for 37 references. In the final analysis, 31 references were included, comprehending 35 cases. Flowchart illustrating the search strategy is shown in Fig. 4. Studies included were either a case report or a case series.
Cases from India, Slovakia, USA and Italy were the most common (19.3 %, 12.9 %, 9.7 % and 9.7 %, respectively). A total of 35 patients were included, corresponding to 17 male and 12 female (the sex of six patients was not informed). Age ranged from a 6-day-old neonate to 88 years old (mean age was 40.3 years). The most common clinical presentation was fever (25 %); 16.1 % were related to catheter infection; 67.7 % had positive blood cultures (54.8 %) or of cerebrospinal fluid (12.9 %).
Only one patient was found to have no previous co-morbidity. Twenty-three patients were immunosuppressed (considering both immunologic disorders and/or use of immunosuppressive agents). Neoplasias were described in 48.4 % of the patients.
Resistance profile of P. laurentii was reported for most cases; one case showed resistance to fluconazole and flucytosine and another to amphotericin B. Amphotericin B was the first choice of treatment for 51.6 % of the patients, followed by fluconazole in 35.5 % of the cases. Fluconazole was the choice for maintenance treatment for a longer period. Cure was achieved in 82.8 % of the patients included on this study after proper treatment. These results are summarized in Table 1.
Summary of systematically reviewed reported cases.
| Reference | Country | Age | Sex | Clinical Presentation | Site of Infection | Co-morbidities | Immunossupression | Neoplasm | Resistance | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asano9, 2014 | Japan | 32 | M | Fever | Peritoneal fluid | IgA nephropathy on peritoneal dialysis | Yes | None | Susceptible to amphotericin B, flucytosine, fluconazole and voriconazole | Voriconazole for 3 months | Cured |
| Averbuch10, 2002 | Israel | 16 | M | Fever | Blood culture | Mestastatic ganglioneuroblastoma | Yes | Yes | Resistance to fluconazole and 5-fluorocytosine | Amphotericin B for 3 weeks | Cured |
| Banerjee11, 2013 | India | 76 | M | Fever, shortness of breath, heart failure | Blood culture | Arterial hypertension, coronary artery disease, previous hemorrhagic stroke | None | None | Susceptible to fluconazole, amphotericin B, voriconazole. Resistance to flucytosine | Amphotericin B for 2 weeks followed by fluconazole for 2 weeks | Cured |
| Bauters12, 2001 | Belgium | 45 | M | Fever | Oropharinx | Erytroleukaemia | Yes | Yes | NR | Amphotericin B for 18 days | Death |
| Bhat2, 2016 | India | 26 | F | Diarrhea | Enteritis | Hodgkin’s lymphoma who underwent autologous hematopoetic stem cell transplant | Yes | Yes | Susceptible to amphotericin B, fluconazole, voriconazole, and 5 flucytosine | Voriconazole for 4 weeks | Cured |
| Calista13, 2015 | Italy | 74 | M | Diarrhea and neutropenia | Enteritis | Hepatitis B, colorectal cancer on chemotherapy | Yes | Yes | None | Amphotericin B for 10 days | Cured |
| Cheng14, 2015 | Taiwan | 88 | F | Fever, neutropenia and abdominal pain | Urine and blood culture | Diffuse large B cell lymphoma on chemotherapy | Yes | Yes | NR | Amphotericin B and flucytosine for 2 weeks | Cured |
| Conti15, 2015 | Italy | 47 | F | Fever associated with weakness in the left lower limb | Lungs | Systemic lupus erythematosus and Sjogren Syndrome, treated with cyclosporine A and corticoids | Yes | None | NR | Initially amphotericin B, switched to fluconazole for 8 months due to toxicity | Cured |
| Ding16, 2018 | Malasya | 35 | F | Fever, supraclavicular mass | Blood culture | Hodgkin lymphoma | Yes | Yes | NR | Fluconazole for 2 weeks | Cured |
| Furman-Kuklinska3, 2009 | Polonia | 39 | NR | Fever | Blood culture | Type I membranoproliferative glomerulonephritis, previous use of prednisone and cyclophosphamide | Yes | None | Susceptible to amphotericin B, voriconazole, itraconazole and fluconazole | Fluconazole for 3 weeks, switched to itraconazole for 4 weeks due to persistence of fungemia | Cured |
| Gupta17, 2018 | India | 6 days | M | Worsening of clinic condition | Blood culture | Premature, low birth weight | None | None | Susceptible to amphotericin B and fluconazole | Amphotericin B for 8 days followed by fluconazole for 24 days | Cured |
| James18, 2000 | Venezuela | 69 | F | Fever and headache | CNS | HIV-negative idiopathic CD4 deffciency | Yes | None | NR | Amphotericin B followed by fluconazole | Cured |
| Johnson19, 1997 | USA | 27 | F | Fever and painful cutaneous nodules | Blood culture | Intravenous drug use, pelvic inflammatory disease, infectious endocarditis and bipolar disorder | None | None | NR | Fluconazole for 4 weeks | Cured |
| Johnson19, 1997 | USA | 27 days | M | Candiduria, fungus balls in both kidneys | Blood culture | Hypoplastic lungs and bilateral hydronephrosis due to posterior urethral valves | None | None | NR | Amphotericin B for 2 weeks | Cured |
| Kamalam20, 1977 | India | 40 | NR | Verrucous nodules around the ankle and left foot | Skin | NR | NR | NR | NR | Potassium iodide for 4 months, switched to amphotericin B due to iodism | Cured |
| Khawcharoenporn5, 2006 | Thailand | 35 | M | Fever, headache | Blood culture and CNS | HIV | Yes | None | NR | Amphotercin B for 14 days followed by fluconazole for 3 months | Cured |
| Kordosis21, 1998 | Greece | 34 | M | Dyspnea, dry cough, weight loss, fever, headache and diplopia | CNS | HIV and Kaposi’s sarcoma | Yes | Yes | NR | Amphotericin B and flucytosine for 2 weeks followed by fluconazole as a maintenance therapy | Cured |
| Krcmery4, 1997 | Slovakia | 17 | NR | Fever | Blood culture | Leukemia, recent bone marrow transplant | Yes | Yes | NR | Fluconazole for 2 weeks | Cured |
| Krcmery22, 1998 | Slovakia | NR | M | Catheter-related | Blood culture | Solid tumor | None | Yes | None | Fluconazole for 10 days | Cured |
| Krcmery22, 1998 | Slovakia | NR | M | Catheter-related | Blood culture | Non-Hodgkin lymphoma, corticoids | Yes | Yes | NR | Amphotericin B for 20 days | Death |
| Kunova23, 1999 | Slovakia | NR | NR | Fever, catheter-related | Blood culture | Neutropenia | Yes | Yes | NR | Amphotericin B for 14 days | Cured |
| Kunova23, 1999 | Slovakia | NR | NR | Fever, catheter-related | Blood culture | Neutropenia | Yes | Yes | NR | Fluconazole for 10 days | Cured |
| Kunova23, 1999 | Slovakia | NR | NR | Fever | Blood culture | Neutropenia | Yes | Yes | NR | NR | Death |
| Lynch24, 1980 | USA | 55 | F | Skin rash and proximal muscle weakness | Lungs | Dermatomiosis, chronic use of corticoids | Yes | None | Susceptible to amphotericin B and flucytosine | Amphotericin B for 6 weeks | Cured |
| Manfredi25, 2006 | Italy | 34 | M | Fever and headache | CNS | Intravenous drug user, HIV | Yes | None | Resistance to both amphotericin B and flucytosine | Fluconazole for 46 days | Cured |
| Martinez26, 2016 | Mexico | 65 | M | Skin ulcer | Skin | Cutaneous leishmaniosis | None | None | NR | Itraconazole | Cured |
| Mittal27, 2015 | India | 30 | F | Post-partum, headache and drowsiness | Blood culture | Recent C-section due to fetal distress | None | None | NR | Amphotericin B | Death |
| Molina-leyva28, 2013 | Spain | 8 | F | Skin lesion in the right forearm | Skin | None | None | None | Susceptible to amphotericin B and fluconazole | Fluconazole for 2 weeks | Cured |
| Neves6, 2015 | Brazil | 42 | M | Fever episodes, severe abdominal pain, weakness and respiratory distress | Blood culture | Cervical cancer treated with chemotherapy and radiotherapy 1 year prior to the hospitalization | None | Yes | Susceptible to amphotericin B and fluconazole | Fluconazole for 22 weeks | Cured |
| Park1, 2017 | Korea | 47 | F | Fever, erythematous papules | Blood culture | Refractory acute myeloid leukemia after allogenic hematopoietic stem cell transplantation, on chemotherapy | Yes | Yes | NR | Amphotericin B for 3 weeks | Cured |
| Rodriguez29, 2012 | Colombia | 3 months | M | Bronquiolitis due to adenovirus, catheter-related | Blood culture | Premature baby with down syndrome | None | None | NR | Amphotericin B | Death |
| Shankar30, 2006 | India | 35 | F | Fever, night sweats, pleuritic chest pain and dyspnea | Peritoneal fluid | HIV, diabetes | Yes | None | NR | Fluconazole for 5 weeks | Cured |
| Simon31, 2005 | Hungary | 9 | M | Enlarged lymph nodes and hepatosplenomegaly | CNS | x-linked hyper-immunoglobulin M syndrome | Yes | None | Susceptible to amphotericin B and fluconazole | Fluconazole for 9 months with tittering of dosage | Cured |
| Sinnott32, 1989 | USA | 13 | F | Abdominal pain and fever | Peritoneal fluid | Chronic renal failure in peritoneal dialysis | Yes | None | NR | Flucytosine and miconazol, switched to amphotericin B for 2 months | Cured |
| Vlchkova-Lashkoska33, 2004 | Slovakia | 51 | M | Skin lesion on the back | Skin and CNS | Alcoholism | None | None | Susceptible to amphotericin B and flucytosine | NR | NR |
M: Male; F: Female; NR: not reported; HIV: Human Immunodeficiency Virus; CNS: Central nervous system.
P. laurentii has a high degree of interspecies heterogeneity and has been divided into phylogenetic groups I and II. Physiologic and biochemical characteristics of the species in the complex are similar. Nevertheless, the species in phylogenetic group I, such as Cryptococcus flavescens and Cryptococcus aureus, can be distinguished from phylogenetic group II by their combination of assimilation patterns of d-glucosamine, Nacetyl-d-glucosamine, DL-lactic acid, 1,2-propanediol and sodium nitrite and vitamin requirements.34Cryptococcus neoformans and P. laurentii share many common traits and structures – the hemolytic capacity of P. laurentii is an intrinsic characteristic that optimizes its infective capacity and increases its growth in blood.35
The likelihood of cryptococcal infection is highly increased in patients with impaired cell-mediated immunity, including lymphoproliferative disorders, HIV infection (CD4 counts < 100 cells/μl) and hematologic malignancies.36 Other risk factors are: use of steroid or chemotherapy,37 organ transplantation, impaired humoral immunity such as hyper-IgM syndrome,38 non-HIV lymphopenia,39 invasive devices40 and direct or indirect exposures to pigeon excreta.41 From our analysis, the presence of invasive catheters, immunosuppression and neoplasms were significant risk factors associated to P. laurentii infection.
P. laurentii has been reported to cause infections in many organ systems.42 The bloodstream and central nervous system were the most common sites of infection, although some other sites such as keratitis43 have been reported. Fever was the most common clinical finding, present in most cases. Choices and duration of treatment for P. laurentii infections depended on the anatomical involvement, host-immune status, and severity of infection. Recommendations regarding treatment for infections are limited, due to the small number of empirically treated cases and the absence of controlled trial data. Amphotericin B alone was used for most treatments, with a high rate of cure (80 %). The most used regimen was an induction period of 14 days followed by maintenance fluconazole, with a cure rate of 75 %. Nonetheless, 10 patients were treated with monotherapy with fluconazole, with a cure rate of 90 %.
A joint clinical guideline published in 2013 by the European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group (ESCMID) and the European Confederation of Medical Mycology (ECMM) for the diagnosis and management of rare invasive yeast infections is the available consensus on how to manage these infections. For non-neoformans and non-gattii Cryptococcus infections it is recommended the use of amphotericin B with or without flucytosine for the induction of CNS and severe infections or fluconazole in a dose over 400 mg daily if demonstrated in-vitro sensitivity. For non-CNS and non-severe infections, 400 mg of daily fluconazole can be used for induction and maintenance treatment, reserving amphotericin B for less azole susceptible species. Due to intrinsic resistance, echinocandins are not recommended.44
Susceptibility testing was reported for only 13 isolates, including our isolate. One was found to be resistant in vitro to amphotericin B and other to fluconazole. Although our isolate was not resistant in vitro to fluconazole, monotherapy failed, justifying a switch to amphotericin B followed by maintenance therapy with fluconazole. Clinical correlations between susceptibility testing results and treatment outcome are lacking.19
The cure rate of the infection was 82.8 %, and the most effective drug was amphotericin B, used in 44.8 % of the cured cases. Although this infection generally occurs in patients with multiple co-morbidities, it does not appear to be very severe, with a high response rate to commonly used therapy for resistant yeast.22,45
A very important concern regarding our reported case must be brought into attention: there is a report of misdiagnosis by Vitek systems, confounding candida species, such as C. parapsilosis, with P. laurentii.7 We do not believe this was the case, since the culture had to be sent to a different lab and regrown for the antifungigram, which confirmed the first and second C. laurentii diagnosis with the same resistance profile and the C. parapsilosis diagnosis in a different system.
In conclusion, P. laurentii, generally considered a non-infective saprobe, may cause relevant fungemia and other infections, especially in immunocompromised and oncologic patients. Central catheters seem to be a particular risk factor for fungemia with this yeast. The main clinical manifestation is fever, blood cultures are useful for diagnosis, and induction treatment with amphotericin B followed by maintenance fluconazole seems to achieve a significant success rate.
Conflict-of-interest statementAll authors have nothing to disclose.
Author contributionsAll authors contributed to study concept and design, and drafting of the manuscript; All authors contributed to acquisition of data, analysis and interpretation of data; Michelin L contributed in revising the final manuscript; Soldera J and Corso LL contributed to statistical analysis; Soldera J contributed to study supervision; all authors contributed to critical revision of the manuscript for important intellectual content.