We report a rare case of Campylobacter fetus bacteremia in a 50-year-old woman following kidney transplantation. Bacteremia was complicated by multivisceral signs such as multiple splenic abscesses, bacterial hepatitis, erythema nodosum and reactive arthritis. Despite a prolonged diagnostic delay, the diagnosis was made on blood culture identification and the global outcome was favorable with adequate antibiotherapy. Reports in the literature describe a high rate of mortality for Campylobacter spp. septicemia, with most patients being immunocompromised. However, Campylobacter spp. has been rarely described in renal transplant patients. Moreover, a splenic septic localization due to Campylobacter spp. has been reported only once to our knowledge. Clinicians should be aware of the diagnostic difficulties related to the frequent negativity of stool samples in C. fetus septicemia, in order to implement a tailored medical strategy. Some data suggest that rapid introduction of adapted antibiotic therapy is associated with a reduction in mortality.
Kidney transplantation is the treatment of choice for many people who have reached chronic end-stage renal failure, increasing both expectations and quality of life. Infections are the most common complications following kidney transplantation and the second leading cause of death in patients with functional grafts and anti-rejection therapy. In these severely immunocompromised patients, clinical and microbiological presentations may show extreme variety, and risk of worsening is higher than in the general population.1 Herein, we report a case of Campylobacter fetus bacteremia in a patient following renal transplantation, complicated by several rare visceral signs. We also propose a review of the literature on previous cases reported for each of the signs.
Case presentationA 50-year-old woman was hospitalized in the nephrology department of our tertiary care center for febrile diarrhea in August 2016. She had a history of chronic renal failure secondary to congenital oligomeganephronic hypoplasia for which she had been transplanted three times. The first two transplants (1987 and 1989) were complicated by rejections leading to hemodialysis in 2006. She received a third transplant in September 2014, with a hyper-immune induction protocol with rituximab and polyvalent immunoglobulins (Thymoglobulin ®). The graft function subsequently stabilized with a nadir creatinine at 1.65 mg/dL. The prevention of transplant rejection was provided by a combination of tacrolimus, mycophenolate mofetil and prednisolone, then mycophenolate mofetil was switched to azathioprine following norovirus diarrhea in December 2014. After this episode, she was treated by four monthly courses of polyvalent immunoglobulins because of allograft glomerulonephritis as shown by graft biopsy. Family history included antecedents of ankylosing spondyloarthropathy in a first- and a second-degree relative. She denied any intoxication, trip abroad, or contagion.
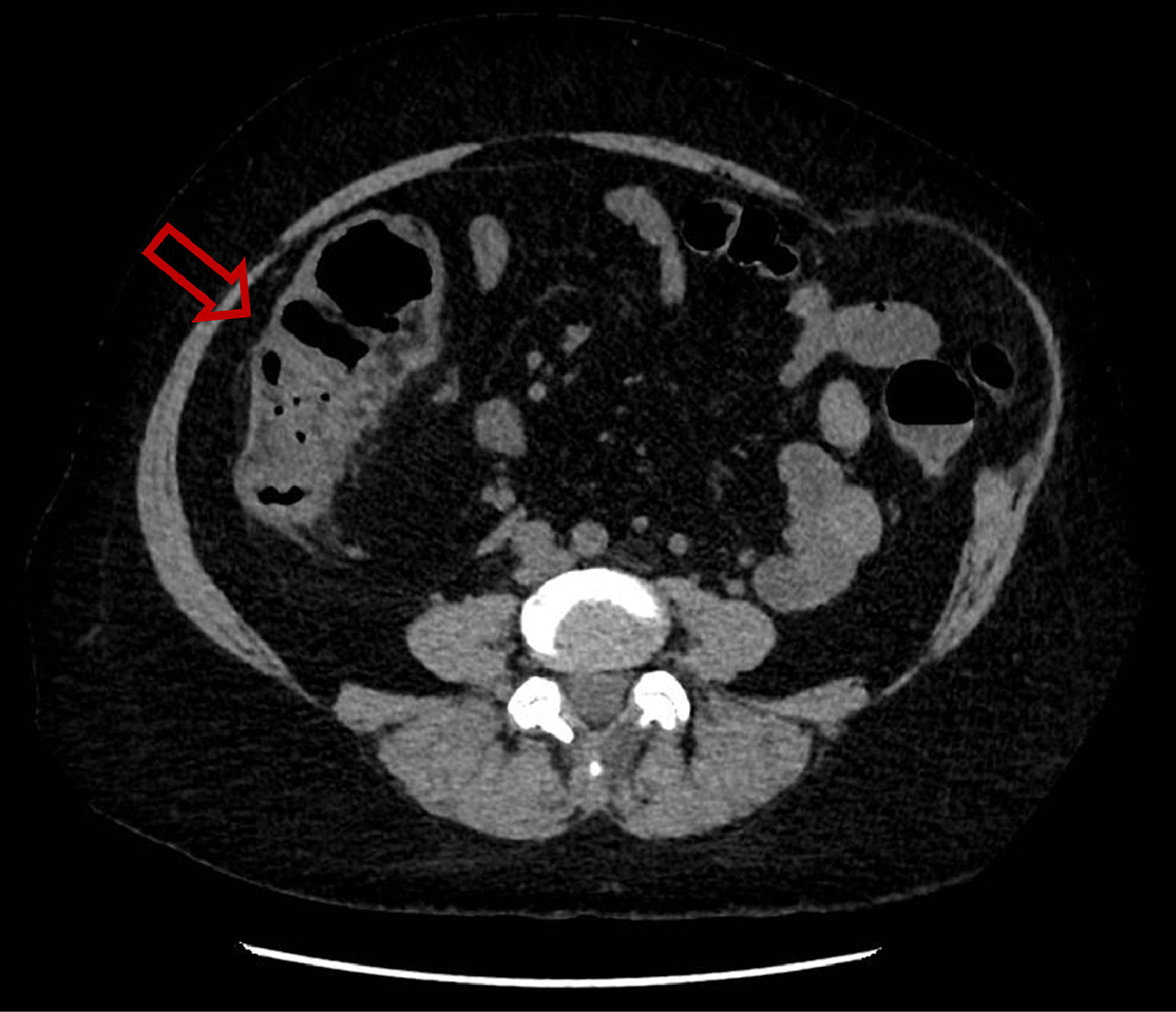
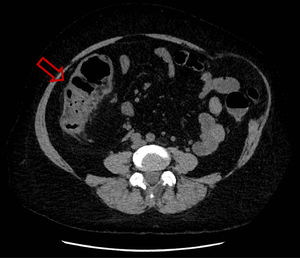
She was hospitalized for the first time because of febrile aqueous diarrhea evolving for 72 hours. A non-injected abdominopelvic computed tomography (CT) scan showed right ileocolitis (Fig. 1). First-line investigations included stool culture, parasitological examination of stools that were negative, a blood culture pair before antibiotic therapy that returned sterile, and a negative viral load for cytomegalovirus (CMV). Then, antibiotic treatment was started with 1 g of intravenous ceftriaxone once a day and 500 mg of oral metronidazole three times a day for five days, allowing the regression of symptoms and the patient was then discharged.
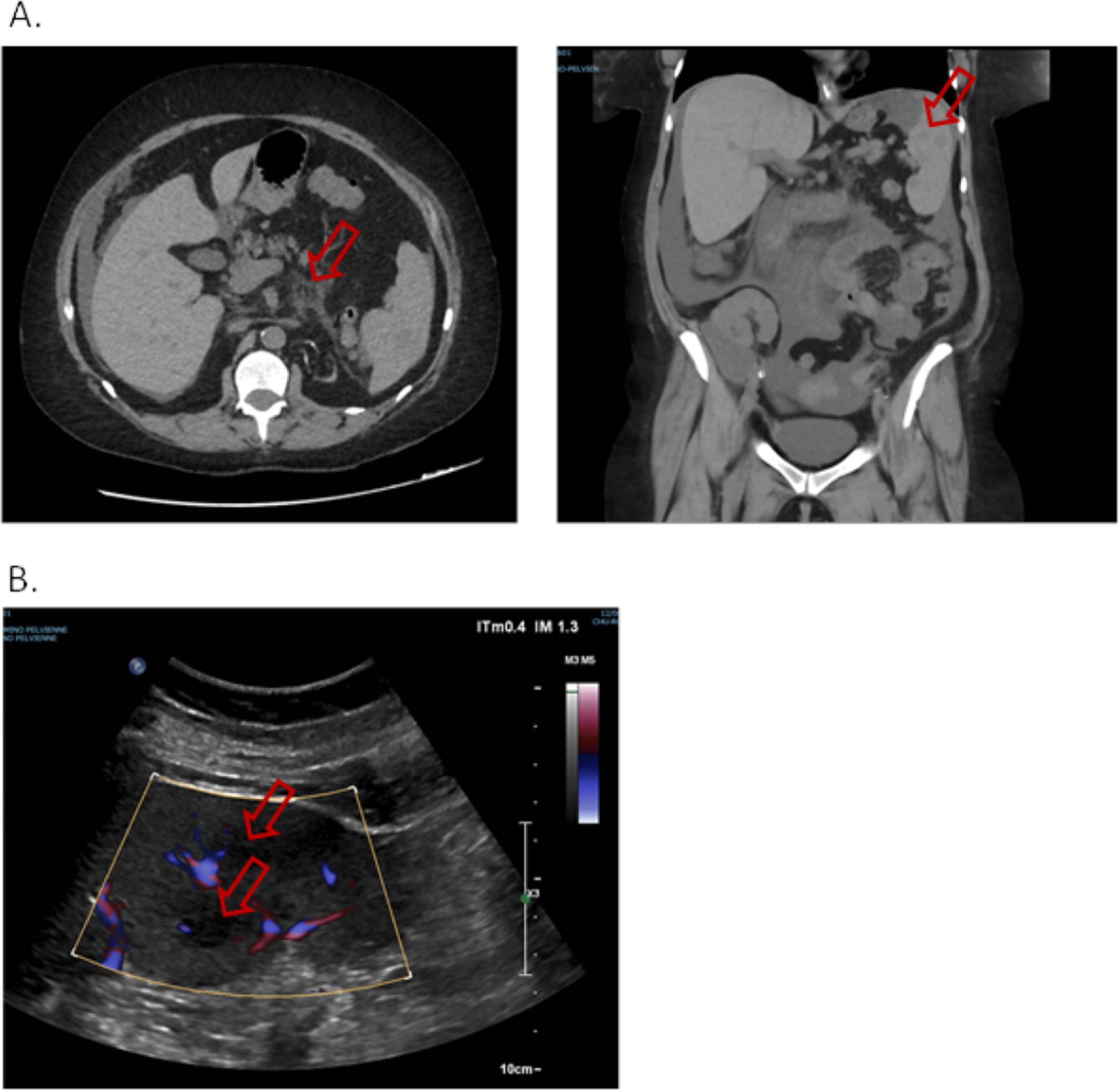
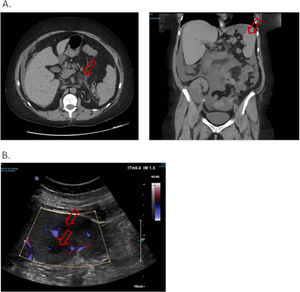
Five days later, she presented to emergency department for abdominal pain. The patient was afebrile, the abdomen flexible. A new non-injected CT scan was performed and found migration of the digestive inflammation site that resulted in duodeno-jejunitis with regression of the right ileocolitis, and a large peritoneal effusion was observed, as well as multiple hypodense splenic lesions, the largest being measured at 30 mm in the lower pole (Fig. 2.A.). The examination was supplemented by a doppler ultrasound which showed hypoechogenic and non-vascularized lesions, some of which presented a target appearance suggestive of candida abscess according to the radiologist's interpretation (Fig. 2.B.). The patient was admitted once more to the nephrology department. A gastroscopy was performed, with no macroscopic characterized abnormality outside atrophic gastritis, and gastric and duodenal biopsies performed came back sterile on standard culture and Sabouraud medium. Six blood cultures were taken and remained sterile, including one before any antibiotics. Empirical treatment was introduced with 4 g of intravenous piperacillin + tazobactam three times a day for 14 days, and oral fluconazole (800 mg on the first day then 200 mg once a day) for six weeks, with adjustment of the dosage of tacrolimus. An abdominal ultrasound examination was performed after the antibiotic treatment, which found a total regression of splenic lesions and peritoneal effusion. The patient was discharged.
A. Non-injected abdominopelvic scan. Duodeno-jejunitis with heterogeneous circumferential wall thickening and densification of fat around the duodenojejunal frame. Peri hepato-splenic, inter-anses, parietal-colic gutters and pouch of Douglas peritoneal effusion of great abundance. Multiple hypodense splenic nodules. B. Splenic Doppler ultrasound. Non-vascularized hypoechoic lesions with Doppler energy, some with a target appearance.
Three weeks later, she consulted again, for oligo-arthritis of both ankles and the right knee, for which treatment with colchicine was introduced with low efficacy. She was re hospitalized in the same unit for etiological assessment. On physical examination, she had lost 7 kg in two months, and was afebrile. There were no more diarrhea. Biologically, we noted lymphopenia (0.46 G/L), C-reactive protein (CRP) at 88 mg/L, mild cytolysis and cholestasis with aspartate aminotransferase (AST) and alkaline phosphatase (ALP) less than twice the upper limit of the normal value, while alanine aminotransferase (ALT) value was in the normal range. Serum creatinine was stable at 3.0 mg/dL. A new pair of blood cultures returned sterile, and trans-thoracic and trans-esophageal ultrasound examinations were normal. An immunological assessment was performed, including anti-nuclear antibodies, native anti-DNA, anti-soluble nuclear antigens, anti-neutrophil cytoplasmic antibodies (ANCA), rheumatoid factor, anti-cyclic citrullinated peptide (CCP), which were all negative. Complement examination (C3, C4) was normal. The left ankle was punctured, and a sero-hematologic fluid was found, examination of which showed predominantly polynuclear cells, while standard culture remained sterile. During hospitalization there was onset of warm erythema discreetly infiltrating the calf and the left tibial crest and both heels, compatible with panniculitis. Conversely, abnormalities of the hepatic assessment increased with cholestasis at three times the normal value and cytolysis (AST, ALT) at more than four times the normal value, without evidence of hepatocellular insufficiency. She had stopped treatment with fluconazole a few days before the onset of liver disturbance and no other drug therapy was introduced during hospitalization. A new etiological assessment was carried out with in particular viral serologies (HIV, HBV, HCV, HAV, HEV) and blood viral loads (EBV, VZV, HSV) without any argument for ongoing viral infection. The anti-smooth muscle and anti-mitochondrial antibodies were negative and a new injected thoracoabdominopelvic CT scan performed was normal. Finally, while a liver biopsy was planned for documentation, a new blood culture performed in the absence of fever returned positive on the aerobic flask for Campylobacter fetus, more than two months after the onset of symptoms. We therefore concluded a diagnosis of bacteremia with C. fetus as the starting point, complicated by uncommon multiple splenic abscesses, reactive arthritis, erythema nodosum and bacterial associated hepatitis. A new treatment was introduced with 1 g of intravenous amoxicillin + clavulanic acid twice a day, which was switched to oral azithromycin (500 mg on the first day, then 250 mg once a day), then a 500 mg dose of oral ciprofloxacin once a day in view of a minimal inhibitory concentration (MIC) at 0.19 mg/L for this molecule. Antibiotics were well tolerated. Immunosuppressive therapy remained unchanged during the entire duration of treatment. Since a Positron Emission Tomography (PET) scan performed during antibiotic therapy eliminated the presence of any infectious vascular pseudoaneurysm, a 14-day treatment duration was decided, then we observed very rapid improvement of all symptoms, and liver function test abnormalities returned to normal. We did not observe any recurrence after a 5-year follow-up.
Discussion and conclusionWe describe an unusual case of C. fetus bacteremia, complicated by multiple splenic abscesses, reactive arthritis, erythema nodosum and associated bacterial hepatitis. Campylobacter spp. is a microaerophilic fastidious Gram-negative bacillus, mainly involved in cosmopolitan diarrheal diseases. The mode of contamination is related to consumption of undercooked meat, and C. fetus has a very varied reservoir (sheep, cattle, poultry, reptile, pork). In Europe, the species is naturally sensitive to beta-lactams associated with beta-lactamase inhibitors as well as macrolides. Cephalosporins are active to a lesser extent and resistance to fluoroquinolones exceeds 30% in some case-series.2–4
Our case has major interest for many reasons. First, C. fetus septicemia is rare, and may be difficult to diagnose, with, in the report, a prolonged diagnostic delay of more than two months. In fact, there are few retrospective cohorts of patients with Campylobacter bacteremia in the literature,2–11 one of the largest being that of Pacanowski et al. (2008). In that series, C. fetus was the most frequently isolated subspecies (53% of cases), as opposed to other observations where C. jejuni was the most often individualized subspecies. This discrepancy may be related to the difficulties of precise identification by the usual laboratory methods,3,8 improvement in recovery of C. fetus through use of automated blood culture systems,2 or predominance of any of the subspecies in elderly or immunocompromised patients.2,3 All cohorts report a high rate of immunosuppression of various origins: solid or hematological neoplasia, chronic liver disease, HIV infection, diabetes or chronic cardiovascular disease.2,4 However, cases of Campylobacter spp. infections have only been described eight times in renal transplant patients, with only two cases of C. fetus.6,10–15 The cohort of Pacanowski et al. also included six transplant patients, but of unspecified sites.2 Two elements contributed to the diagnostic delay in this observation. On the one hand, the first presentation was atypical, with multiple splenic lesions suggesting candida etiology. On the other hand, Campylobacter spp. is fastidious to cultivate, with very slow growth in standard conditions, and in this situation the results of microbiology were delayed because of an empiric antibiotic active on C. fetus (namely ceftriaxone during the first hospitalization and piperacillin + tazobactam during the second hospitalization). However, a standard stool culture and a pair of blood cultures were performed before initiation of any antimicrobial treatment and remained negative, and the bacteremia appeared more than a month after stopping antibiotic therapy. Interestingly, stool culture is less often positive in C. fetus bacteremia than in other subspecies bacteremia,2 and C. fetus has never been found in a series of Campylobacter spp. non-bacteremic infection with positive stool culture.9 These data may suggest rapid digestive clearance and superior digestive translocation ability for fetus subspecies or differences in growth capacities on selective media used for digestive pathogens identification. In addition, distant septic complications are more common for fetus than for other subspecies, including more endovascular infections and cellulitis.2
Secondly, this case of C. fetus septicemia was complicated by several uncommon infectious signs. To our knowledge, only one case of Campylobacter spp. splenic abscess has been described previously, with a single bulky presentation in a non-bacteremic young patient with no evidence of immunosuppression.16 In our case, the assessment of splenic lesion vascularization on doppler ultrasound made it possible to rule out a diagnosis of pseudoaneurysm. Despite the absence of injection of iodinated contrast medium, we retained a diagnosis of multiple splenic abscess related to Campylobacter infection. The disappearance of all lesions after 14 days of treatment with piperacillin + tazobactam strongly supports this hypothesis. Cases of hepatitis associated with Campylobacter spp. are rarely described, with only one for C. fetus in adults.17 Usually, these cases of hepatitis are cytolytic, associated with hepatomegaly and benign with consistantly favorable evolution under treatment. Liver biopsy when performed shows a specific polymorphonuclear infiltrate sometimes accompanied by hepatocyte necrosis, and Campylobacter is never found in the microbiological analysis of hepatic samples. Hepatic involvement is thought to be related to the production of hepatotoxic factors by the bacteria, which causes aseptic hepatitis.
Thirdly, our case is also remarkable because of the association of several dysimmune complications associated with Campylobacter infection, with multivisceral presentation. Cases of reactive arthritis are common in Campylobacter spp., occurring in 0.9-5% of cases, but have rarely been described after fetus subspecies infection.18,19 An average delay of one to four weeks is generally reported between the beginning of the digestive and articular symptoms, but some cases have been described with more than one year of delay. In our observation, skin lesions were more related to erythema nodosum than to cellulitis due to topography evocative of a bilateral lower extremity. The occurrence of erythema nodosum is classic in the aftermath of a Campylobacter gastrointestinal infection but in fact very rarely described, with only one case for C. fetus species.20 These immunological complications in a highly immunocompromised patient are quite remarkable.
Finally, the evolution of our case was favorable under macrolide then fluoroquinolone antibiotic therapy, whereas prolonged intravenous betalactamine treatment was not successful, although the antibiogram data suggested the in vitro efficacy of beta-lactams associated with beta-lactamase inhibitor. This can be explained by the lack of penetration of the latter at the digestive Peyer plates. Campylobacter spp. bacteremia mortality ranges from 2 to 28% in retrospective series,2–11 with several series reporting a death rate greater than 15%.2,3,5 The lack of appropriate antibiotic treatment is found as an independent factor of mortality,2 although the evolution seems to be favorable for most untreated or inadequately treated patients.7,11
As a conclusion, Campylobacter spp. bacteremia is predominant in immunocompromised patients with infections of digestive origin and is responsible for significant mortality and potentially disabling immunological complications that clinicians should be aware of. Very few observations describe these events for the fetus subspecies. Our case is also characterized by the presence of multiple splenic abscesses and a regressive liver injury under treatment. Microbiological diagnosis may be a challenge given the frequent negativity of stool cultures and the difficulties in individualizing the bacteria on blood culture, especially in cases of concomitant antibiotic prescription. However, microbiological diagnosis remains crucial insofar as antibiotic therapy adapted to the antibiogram data seems to reduce mortality in this context.
Consent for publicationThe patient has given signed consent for publication. Written consent is available by request.
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.