Several therapies have been used or proposed for the treatment of COVID-19, although their effectiveness and safety have not been properly evaluated. The purpose of this document is to provide recommendations to support decisions about the drug treatment of outpatients with COVID-19 in Brazil.
MethodsA panel consisting of experts from different clinical fields, representatives of the Brazilian Ministry of Health, and methodologists (37 members in total) was responsible for preparing these guidelines. A rapid guideline development method was used, based on the adoption and/or adaptation of recommendations from existing international guidelines combined with additional structured searches for primary studies and new recommendations whenever necessary (GRADE-ADOLOPMENT). The rating of quality of evidence and the drafting of recommendations followed the GRADE method.
ResultsTen technologies were evaluated, and 10 recommendations were prepared. Recommendations were made against the use of anticoagulants, azithromycin, budesonide, colchicine, corticosteroids, hydroxychloroquine/chloroquine alone or combined with azithromycin, ivermectin, nitazoxanide, and convalescent plasma. It was not possible to make a recommendation regarding the use of monoclonal antibodies in outpatients, as their benefit is uncertain and their cost is high, with limitations of availability and implementation.
ConclusionTo date, few therapies have demonstrated effectiveness in the treatment of outpatients with COVID-19. Recommendations are restricted to what should not be used, in order to provide the best treatment according to the principles of evidence-based medicine and to promote resource savings by aboiding ineffective treatments.
Worldwide, as of February 20th, 2022, the World Health Organization (WHO) had reported over 422 million confirmed cases and over 5.8 million deaths from COVID-19.1 In Brazil, as of February 24th, 2022, 28,484,890 cases and 646,419 deaths from COVID-19 had been confirmed, with an estimated cumulative incidence rate of 13,554.7 cases per 100,000 population and a cumulative mortality rate of 307,6 deaths per 100,000 population.2
Most frequently, people with COVID-19 have mild clinical manifestations, developing symptoms such as fever, dry cough, and fatigue, with self-limited resolution. However, approximately 14% of COVID-19 cases progress to severe disease and may require oxygen therapy or hospitalization, and 5% are admitted to an Intensive Care Unit (ICU).1
Given the high morbidity and mortality from COVID-19 in a short period, health care systems all around the world face the challenge of reorganizing to meet the demands imposed by the pandemic. Also, there are uncertainties and variations in clinical practice,3 and scientific knowledge is not able to evolve at the pace required by health care needs. Therefore, healthcare services and health professionals are needed to ensure that patients are cared for in a timely manner, leading to the achievement of better clinical outcomes.
Medical societies (Brazilian Association of Emergency Medicine, Brazilian Association of Intensive Care Medicine, Brazilian Medical Association, Brazilian Society of Angiology and Vascular Surgery, Brazilian Society of Infectious Diseases, Brazilian Thoracic Society, Brazilian Society of Rheumatology, together with the Brazilian Ministry of Health) have developed guidelines for the pharmacological treatment of patients hospitalized with COVID-19.4 As most therapies have proved ineffective, their use is not recommended. Recommendations have been made for the use of corticosteroids in patients requiring supplemental oxygen as well as anticoagulation therapy in hospitalized patients, outside the intensive care setting. Additionally, the benefit of tocilizumab has been recognized, especially for patients with a marked clinical deterioration but not on invasive mechanical ventilation.
Given the continued importance of COVID-19 and the need for adequate prehospital management, this document aims to guide clinical practice regarding the pharmacological treatment of outpatients with COVID-19.
MethodsThe method used in this document was rapid guideline development, based on the adoption and/or adaptation of recommendations from existing international guidelines combined with additional searches for primary studies and new recommendations whenever necessary (GRADE-ADOLOPMENT).5,6
Organization, panel composition, and guideline developmentThe guideline development group consisted of an expert panel coordinated by the Department of Technology Management and Incorporation and Health Innovation, Office for Science, Technology, Innovation, and Strategic Inputs, Brazilian Ministry of Health (DGITIS/SCTIE/MS). The expert panel included family medicine physicians, internists, emergency physicians, intensive care physicians, vascular and endovascular surgeons, infectious disease specialists, pulmonologists, endocrinologists, and representatives of the Brazilian Ministry of Health, universities, centers of excellence, and medical societies. The following medical societies participated in the development of these guidelines and endorse the present recommendations: Brazilian Association of Emergency Medicine (ABRAMEDE); Brazilian Medical Association (AMB); Brazilian Society of Angiology and Vascular Surgery (SBACV); Brazilian Society of Geriatrics and Gerontology (SBGG); Brazilian Society of Infectious Diseases (SBI); Brazilian Society of Family and Community Medicine (SBFMC); Brazilian Thoracic Society (SBPT).
From late June to October 2021, the Steering Committee held 10 online video conferences with the experts to prepare and discuss the guidelines, until a consensus was achieved. The Steering Committee members and methodologists did not interfere in the development of guidelines by the experts.
Identification of clinical questionsTo identify the clinical questions of interest, the technologies evaluated in nine national and international guidelines for the treatment of outpatients with suspected or confirmed COVID-19 were reviewed. The most relevant technologies with greatest practice variation in Brazil were selected, provided they had regulatory approval for outpatient use. In addition to those documents, a position statement issued by the Pan American Health Organization (PAHO) was considered in the discussions.
The following technologies were evaluated: anticoagulants, azithromycin, monoclonal antibodies, budesonide, colchicine, chloroquine and hydroxychloroquine, systemic corticosteroids, ivermectin, nitazoxanide, and convalescent plasma.
Evidence synthesisRecommendations, evidence profiles, and GRADE (Grading of Recommendations Assessment, Development and Evaluation) domains were extracted from evidence-to-decision tables through the eCovid RecMap platform, and the original documents were consulted whenever necessary.7 The identified guidelines were reviewed, and their recommendations were extracted. The following guidelines and evidence synthesis documents were used in the guideline development process: Brazilian Association of Intensive Care Medicine (AMIB), Brazilian Society of Infectious Diseases (SBI), and Brazilian Thoracic Society (SBPT): “Diretrizes para o tratamento farmacológico da COVID-19. Consenso da Associação de Medicina Intensiva Brasileira, da Sociedade Brasileira de Infectologia e da Sociedade Brasileira de Pneumologia e Tisiologia”;8 Brazilian Medical Association (AMB): “COVID-19: Summary Recommendations”;9 Australian National COVID-19 Clinical Evidence Taskforce: “Caring for people with COVID-19 - Supporting Australia's healthcare professionals with continually updated, evidence-based clinical guidelines”;10 European Respiratory Society (ERS): “Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline”;11 Infectious Diseases Society of America (IDSA): “Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19”;12 National Institute for Health and Care Excellence (NICE): “COVID-19 rapid guideline: managing COVID-19”;13 National Institutes of Health (NIH): “COVID-19 Treatment Guidelines”;14 World Health Organization (WHO): “Therapeutics and COVID-19 – living guideline”;15 Pan American Health Organization (PAHO): “Ongoing Living Update of COVID-19 Therapeutic Option – Summary of Evidence”;16 A Position Paper of the German Society for Applied Allergology (AeDA) and the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO).17
Knowledge about COVID-19 has evolved over the past 12 months as a result of collaborative efforts from several countries and research groups, with the development of randomized clinical trials evaluating potential drugs for disease treatment. Therefore, the guideline panel obtained evidence exclusively from randomized clinical trials, which are deemed the most robust and adequate studies to ascertain drug efficacy and safety. Thus, the PubMed and medRxiv databases were searched to identify randomized clinical trials evaluating the use of each of the technologies for outpatient treatment. A detailed description of the methodology (including search strategy, screening and study selection, data collection and analysis, risk of bias and certainty of evidence), recommendations from international guidelines, evidence synthesis, and additional rationale for the judgment made are presented in supplementary material.
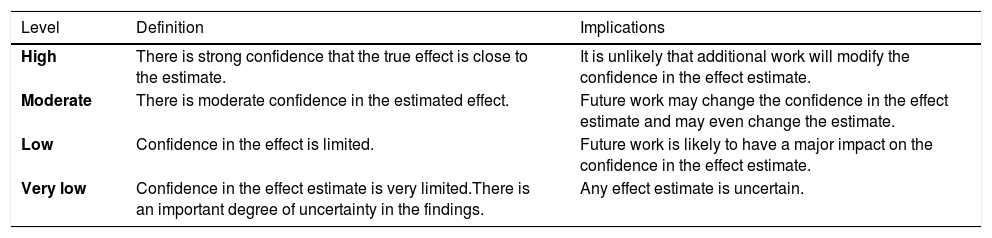
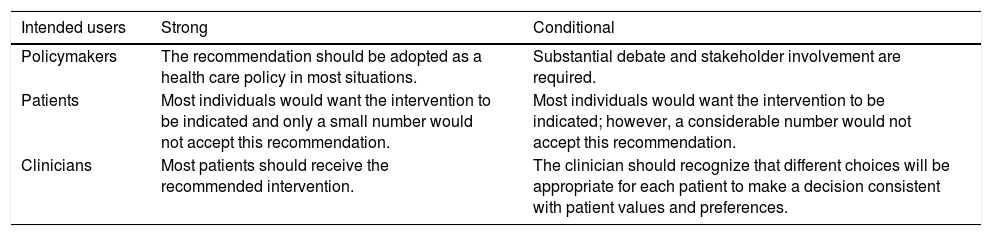
Assessing certainty in evidence and developing recommendationsFor developing recommendations, evidence of benefits and risks, quality of evidence, costs and use of resources, feasibility, and aspects related to equity, patient values and preferences, and acceptability were considered. The GRADE system was used to assess certainty in evidence, which is rated into four levels: high, moderate, low, and very low (Table 1). For each recommendation, the direction of the course of action was discussed (whether to perform or not the proposed action), and the strength of recommendation was defined as strong or conditional according to the GRADE system (Table 2). Supplementary material shows evidence profile tables with the certainty of evidence and the strength of recommendation, together with the corresponding interpretation, for each of the evaluated technologies.18,19 The terminology “we recommend” and “we suggest” denote different degrees of emphasis on the strength of recommendation, as shown below: “We recommend” represents a strong recommendation, which should be incorporated as a routine practice, either for or against the use of a given intervention; “We suggest” represents a conditional recommendation, which is applicable to most situations; however, either due to lack of robust evidence or to expected variation in treatment effectiveness, other approaches may be justifiable.
Levels of evidence according to the GRADE system.
Source: Diretrizes metodológicas: Sistema GRADE – Manual de graduação da qualidade da evidência e força de recomendação para tomada de decisão em saúde/Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Ciência e Tecnologia. – Brasília: Ministério da Saúde, 2014.
Implications of the strength of recommendation for clinicians, patients, and policymakers.
Source: Diretrizes metodológicas: Sistema GRADE – Manual de graduação da qualidade da evidência e força de recomendação para tomada de decisão em saúde/Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Ciência e Tecnologia. – Brasília: Ministério da Saúde, 2014.
These Brazilian guidelines address adult male and female patients with suspected or confirmed diagnosis of SARS-CoV-2 infection. These guidelines do not cover specific recommendations for pregnant women.
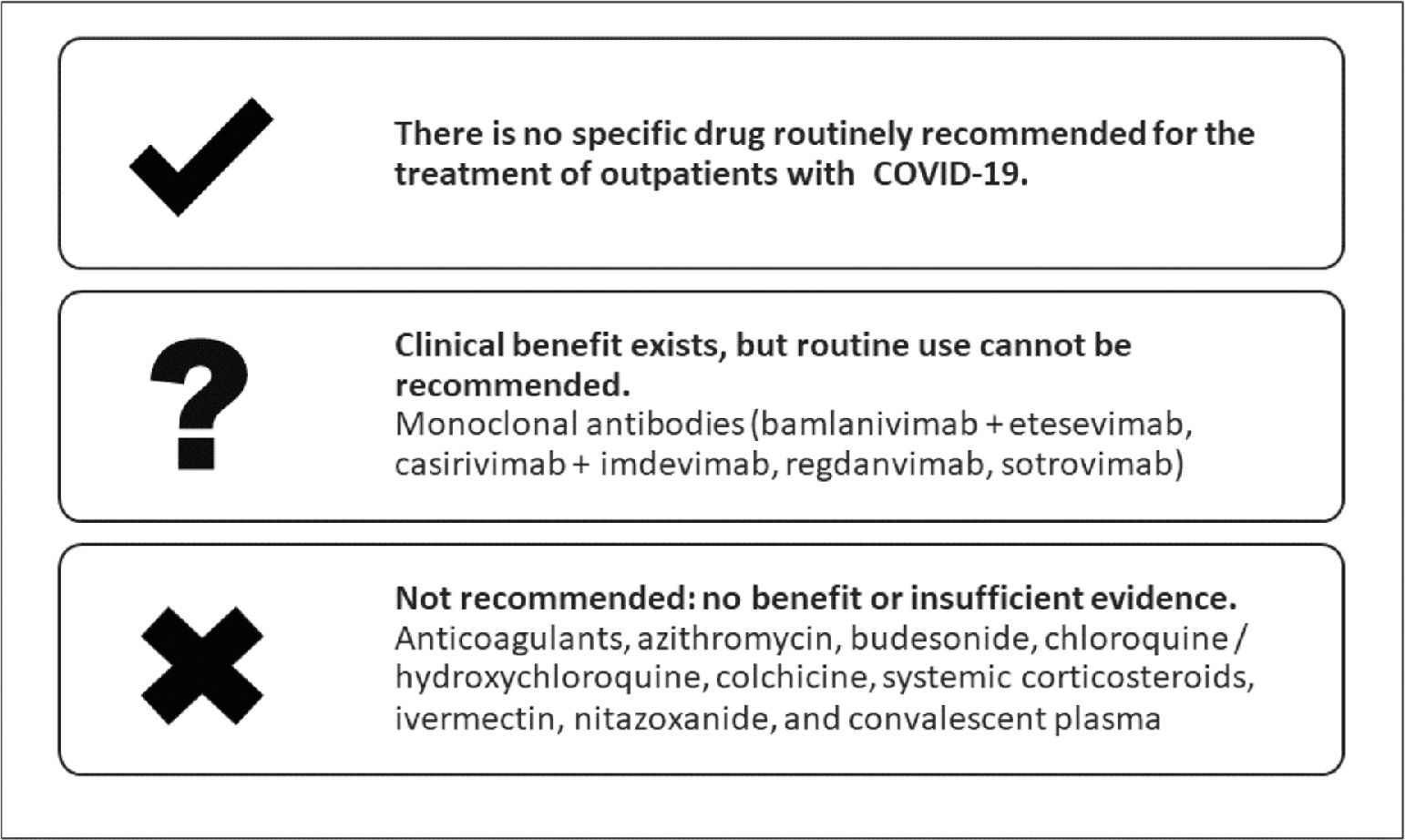
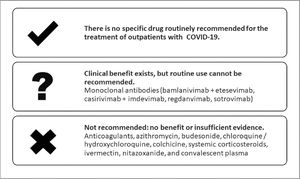
ResultsTen recommendations were made. The recommendations are summarized in Table 3 and in Fig. 1.
Summary of recommendations made by the panel.
Source: The authors.
Below we present the recommendations, the rationale for decision-making, and, when relevant, considerations for implementation. Detailed information on the evidence supporting each recommendation is shown in supplementary material.
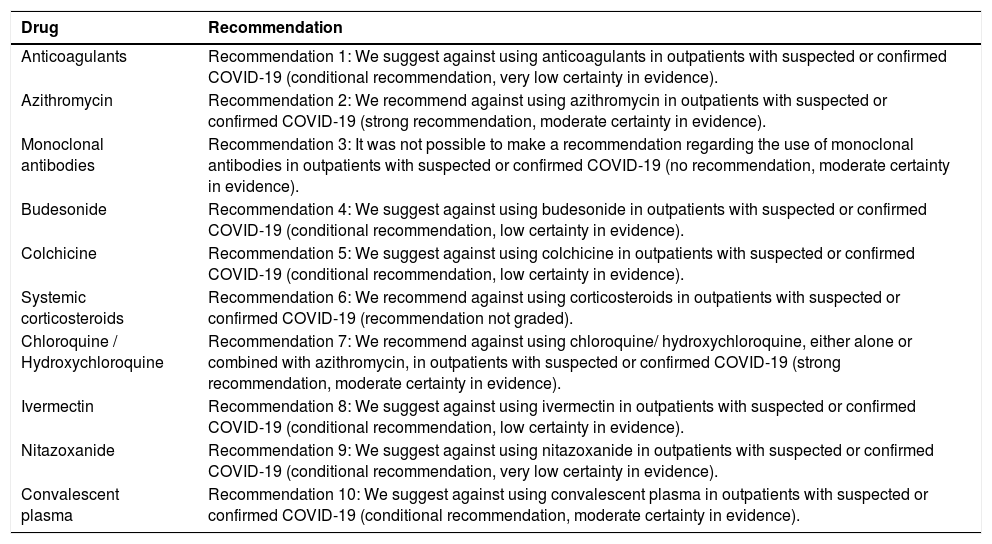
AnticoagulantsRecommendation 1: We suggest against using anticoagulants in outpatients with suspected or confirmed COVID-19 (conditional recommendation, very low certainty in evidence).
Rationale for recommendation: The panel concluded that there is uncertainty about the benefit of using anticoagulants in patients with suspected or confirmed COVID-19 undergoing outpatient treatment; additionally, anticoagulation is associated with an increased risk of bleeding events, which can be even more challenging in an outpatient setting, where closer monitoring to minimize anticoagulation risks is sometimes unfeasible. Also, the only randomized study identified evaluated sulodexide, a drug not available in Brazil, thus being used as indirect evidence for other drugs. Therefore, we understand that there is no basis for indicating routine anticoagulation therapy at this time, and this recommendation should be revised after publication of phase 3 studies, such as the PREVENT-HD evaluating rivaroxaban, and the ACTIV4-outpatients and the APOLLO evaluating apixaban.20-22 These recommendations are in line with the guideline recommendations identified.
General considerations for implementation: The panel concluded that there is no confirmed benefit of the use of anticoagulants in patients with COVID-19; additionally, anticoagulation is associated with an increased risk of bleeding events. This can be even more challenging in an outpatient setting, where closer monitoring to minimize anticoagulation risks is sometimes unfeasible; the recommendation is applicable to prophylactic, intermediate, or therapeutic doses of anticoagulants, regardless of the route of administration (oral or parenteral); there is no indication for D-dimer testing to guide the use of anticoagulants; anticoagulants should be maintained or initiated in patients with a specific clinical indication (e.g., atrial fibrillation, venous thromboembolism); this recommendation does not apply to the use of anticoagulants in post-discharge management of hospitalized patients with COVID-19.
AzithromycinRecommendation 2: We recommend against using azithromycin in outpatients with suspected or confirmed COVID-19 (strong recommendation, moderate certainty in evidence).
Rationale for recommendation: The panel concluded that evidence shows no benefit of the use of azithromycin in patients with suspected or confirmed COVID-19 undergoing outpatient treatment. The drug was not recommended by international guidelines.
General considerations for implementation: Azithromycin can be used when bacterial infection is present or suspected, according to institutional or local protocols of antimicrobial use.
Monoclonal antibodiesRecommendation 3: It was not possible to make a recommendation regarding the use of monoclonal antibodies in outpatients with suspected or confirmed COVID-19 (no recommendation against or for the intervention performed, moderate certainty in evidence).
Rationale for recommendation: The panel concluded that monoclonal antibodies are effective in the treatment of outpatients with suspected or confirmed COVID-19, reducing hospitalization and time to symptom improvement when started early and in patients at high risk for COVID-19. However, there is uncertainty about the benefit of the drug, which also has a high cost, with limitations of availability and implementation. The National Committee for Health Technology Incorporation (CONITEC) should reassess technology incorporation based on current findings, addressing evidence regarding costs, implementation feasibility, equity, and access to drug. Because of these factors, it was not possible to recommend the use of monoclonal antibodies in patients with suspected or confirmed COVID-19 undergoing outpatient treatment.
General considerations for implementation: Currently, the following monoclonal antibodies have regulatory approval for use in patients with COVID-19 in Brazil: bamlanivimab+etesevimab, casirivimab+imdevimab, regdanvimab, and sotrovimab; the recommendation panel found that monoclonal antibodies are effective in the treatment of outpatients with COVID-19, as they reduced hospitalization and time to symptom improvement when started early in the course of disease manifestations and in patients at high risk for COVID-19; there is uncertainty about the benefit of using these drugs: studies evaluated only patients not vaccinated against COVID-19; thus, the benefit is more uncertain in a setting where the majority of the high-risk population is currently vaccinated. Also, given that clinical studies were conducted in places with different profiles of new coronavirus variants, drug benefit is uncertain in Brazil, where there is a predominance of Gamma, Delta and Omicron variants; the drug can be considered in unvaccinated patients (or with an indication for a booster dose, not having received it yet) and at high risk of disease progression: age over 65 years, overweight or obesity, chronic kidney disease, diabetes, cardiovascular disease, hypertension, chronic lung disease, sickle cell anemia, neurodevelopmental disorders (e.g., cerebral palsy), and medical device dependence (e.g., gastrostomy, tracheostomy); it should preferably be administered within the first three days of symptoms; there is still no adequate evidence on the maintenance of the efficacy of monoclonal antibodies to the different new coronavirus variants; bamlanivimab showed a reduced in vitro effect for the Gamma, Delta and Omicron variants, while casirivimab and imdevimab remained active against the Delta variant; the intervention has a high cost, with limitations of availability and implementation, and the drug is authorized for hospital use only, which is a logistical challenge and increases barriers to drug adherence and access; monoclonal antibodies were previously evaluated by CONITEC (casirivimab+imdevimab and bamlanivimab+etesevimab), and their incorporation was not recommended at that time, thus they are not available via Brazilian Unified Health System (SUS); CONITEC should reassess their incorporation based on current findings, addressing evidence regarding costs, implementation feasibility, equity, and access to drug.
BudesonideRecommendation 4: We suggest against using budesonide in outpatients with suspected or confirmed COVID-19 (conditional recommendation, low certainty in evidence).
Rationale for recommendation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use in patients with suspected or confirmed COVID-19 undergoing outpatient treatment. The studies available to date are all open label, with benefit being demonstrated for subjective outcomes. Also, studies generally evaluated patients at higher risk, with no evidence for low-risk patients (young and without comorbidities). Thus, a conditional recommendation against the use of budesonide is presented.
General considerations for implementation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use in outpatients with COVID-19. The clinical trials available to date are all open label, with benefit being demonstrated for subjective outcomes. Also, studies generally evaluated patients at higher risk, with no evidence for low-risk patients (young and without comorbidities); patients with other clinical indications for budesonide (e.g., asthma) benefit from its initiation or maintenance.
ColchicineRecommendation 5: We suggest against using colchicine in outpatients with suspected or confirmed COVID-19 (conditional recommendation, low certainty in evidence).
Rationale for recommendation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use.
General considerations for implementation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use.
Systemic corticosteroidsRecommendation 6: We recommend against using corticosteroids in outpatients with suspected or confirmed COVID-19 (recommendation not graded).
Rationale for recommendation: The panel concluded that there is no evidence to support the use of systemic corticosteroids in patients with suspected or confirmed COVID-19 undergoing outpatient treatment, and they should not be used.
General considerations for implementation: no randomized trials evaluating the effectiveness of systemic corticosteroids in outpatients with COVID-19 were found. Indirect evidence from hospitalized patients not requiring supplemental oxygen showed no benefit, with a potentially increased risk of mortality with the use of corticosteroids in this population; the recommendation is applicable to systemic corticosteroids (oral or parenteral); patients with other indications for corticosteroids (e.g., exacerbated asthma or chronic obstructive pulmonary disease, previous use due to rheumatologic diseases, pulmonary maturation in pregnant women) should use them according to clinical indication.
Chloroquine/hydroxychloroquineRecommendation 7: We recommend against using chloroquine/hydroxychloroquine, either alone or combined with azithromycin, in outpatients with suspected or confirmed COVID-19 (strong recommendation, moderate certainty in evidence).
Rationale for recommendation: The panel concluded that evidence shows no benefit of hydroxychloroquine or chloroquine in patients with suspected or confirmed COVID-19 undergoing outpatient treatment, with an increase in adverse events being observed. The drug was not recommended by any of the identified guidelines.
General considerations for implementation: Chloroquine and hydroxychloroquine should not be used, regardless of the route of administration (oral, inhaled, or others); patients on chloroquine or hydroxychloroquine due to other health conditions (eg, rheumatologic diseases, malaria) should continue its use.
IvermectinRecommendation 8: We suggest against using ivermectin in outpatients with suspected or confirmed COVID-19 (conditional recommendation, low certainty in evidence).
Rationale for recommendation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use.
General considerations for implementation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use.
NitazoxanideRecommendation 9: We suggest against using nitazoxanide in outpatients with suspected or confirmed COVID-19 (conditional recommendation, very low certainty in evidence).
Rationale for recommendation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use.
General considerations for implementation: The panel concluded that, although benefit cannot be ruled out and the drug is relatively safe, current evidence is insufficient to indicate its routine use.
Convalescent plasmaRecommendation 10: We suggest against using convalescent plasma in outpatients with suspected or confirmed COVID-19 (conditional recommendation, moderate certainty in evidence).
Rationale for recommendation: The panel concluded that there is insufficient evidence to indicate the use of convalescent plasma currently, with studies showing divergent results.
General considerations for implementation: There are still uncertainty about evidence regarding specific, high-risk populations, such as immunosuppressed and transplanted patients.
DiscussionThe recommendations made in this guideline were based on evidence from previous rapid guidelines and systematic reviews, carefully evaluated by an expert panel (9 Brazilian Ministry of Health representatives, 23 clinical experts, and 5 methodologists) using the GRADE-ADOLOPMENT methodology for judging and drafting recommendations.5,6,23 Thus, 10 recommendations were prepared, and most of them advise against the use of the evaluated technologies. The expert panel's decision to contraindicate the use of these drugs is consistent with the findings of international guidelines, which also contraindicate clinical treatment for COVID-19 with anticoagulants,10,14 azithromycin,10,13,14 budesonide,10 colchicine,10,13 corticosteroids,10,13-15 hydroxychloroquine/chloroquine alone or combined with azithromycin,10,11,14,15 ivermectin,10,12,15 nitazoxanide,10 or convalescent plasma.10,12,14 Additionally, there are two guidelines developed in Brazil that also contraindicate the use of some of these drugs.8,24
In epidemic situations when no clinical treatment with consolidated effectiveness is available, clinicians tend to indicate drugs based on the results of observational studies, which may have important limitations, or preclinical studies.25 Some clinical treatments that were used in previous epidemics had questionable or reduced benefit, as was the case with oseltamivir during the 2009 swine flu epidemic, for example. In the 2014 Ebola epidemic, multiple interventions were tested, including chloroquine, hydroxychloroquine, favipiravir, biological drugs, and convalescent plasma, none of which had their safety or effectiveness proven.26 Thus, developing documents that synthesize the findings in the scientific literature is of great importance to guide the best possible evidence-based clinical practice.
The understanding of COVID-19 and its treatment has evolved significantly over the past two years, based on collaborative efforts of several countries and research groups, which have developed randomized clinical trials to assess the effects of the main candidate technologies for the treatment of COVID-19. In the first version of the Brazilian guidelines, published in May 2020, most evidence consisted of results of observational studies, with no publications from the main research groups, such as RECOVERY, SOLIDARITY, COALIZÃO, TOGETHER, and PRINCIPLE.8,27-30 Despite some advances, few pharmacological therapies have proven to be effective in the outpatient treatment of COVID-19. With the exception of monoclonal antibodies that showed some benefit, other therapies showed no significant benefit in preventing clinically relevant outcomes, such as hospitalization, progression to mechanical ventilation, and mortality. Bamlanivimab+etesevimab, casirivimab+imdevimab, regdanvimab, and sotrovimab have a suggested clinical benefit in patients at high risk of progression to severe disease; however, it is not possible to make a recommendation in favor of using these drugs because of their high cost, limited experience of use, uncertainty regarding effectiveness, and unavailability in the health care system. There is uncertainty about the benefit of anticoagulants, budesonide, colchicine, ivermectin, nitazoxanide, and convalescent plasma in outpatients, which limits the use of these drugs to randomized clinical trials to assess efficacy and safety. Thus, they are not currently indicated for outpatient treatment of COVID-19. Azithromycin and hydroxychloroquine showed no clinical benefit and therefore should not be used in the outpatient treatment of patients with suspected or confirmed COVID-19. Some emerging therapies include molnupiravir, nirmatrelvir/ritonavir, fluvoxamine, and AZD7442, which were not evaluated in this document. Molnupiravir is the first oral antiviral drug developed for treating COVID-19 that showed positive results in nonhospitalized patients with mild-to-moderate COVID-19.31 Preliminary results demonstrated an approximate 50% reduction in the risk of hospitalization (29-day follow-up).32 More recently, preliminary data regarding the use of nirmatrelvir/ritonavir showed an 80% reduction in hospitalizations.33 Fluvoxamine is a selective serotonin reuptake inhibitor indicated for treating depression, obsessive-compulsive disorders, and other health conditions.34 A recent study demonstrated the benefit of fluvoxamine in reducing the need for hospitalization, defined as either retention for more than six hours in an emergency setting or transfer to a tertiary hospital in patients at high risk of developing severe COVID-19.35 Tixagevimab+cilgavimab consists of a combination of monoclonal antibodies, probably with similar effectiveness, and has the advantage of being delivered by a intramuscular injection.36
These guidelines covered only recommendations for outpatients with COVID-19. In the Brazilian Guidelines for the pharmacological treatment of patients hospitalized with COVID-19 we provided 16 recommendations related to 12 drugs.4 In those guidelines we recommended the use of corticosteroids (preferentially dexamethasone) for patients using supplemental oxygen. Also, suggested the use heparin or enoxaparin in therapeutic doses in noncritical patients (those with no need for vasoactive drugs, renal replacement therapy, high-flow nasal cannula, noninvasive ventilation, or invasive mechanical ventilation) hospitalized with COVID-19. In addition, we acknowledge the benefit of tocilizumab for patients using noninvasive ventilation or high-flow nasal cannula; however, it is not possible to recommend it, as this use is off label and there are uncertainties regarding access to the drug that affect the ability to meet the potential demand. Although remdesivir has potential clinical benefit in this population, its costs and uncertainty related to its effectiveness resulted in a suggestion against its routine use. The effectiveness of most therapies in the treatment of COVID-19 in hospitalized patients was not proved; thus, azithromycin, antimicrobials, casirivimab plus imdevimab, chloroquine or hydroxychloroquine, colchicine, convalescent plasma, ivermectin, lopinavir/ritonavir, and remdesivir were not recommended in those guidelines.4 Some other interventions may be effective in hospitalized patients with COVID-19. Tofacitinib in a phase II randomized study showed 37% reduction on the risk of death or respiratory failure.37 Baricitinib reduced 38% the risk of mortality compared to placebo.38
Demonstrating the technologies that should not be used in clinical practice is also of great importance for patient care, particularly in the context of COVID-19, as clinicians worldwide have used technologies without proven effectiveness, especially in the early stages of the pandemic.11 Thus, the objectives of this guideline were to improve the quality of patient care and to help standardize care in different health care settings and systems that serve patients who are at home or who attend outpatient clinics.
With this guideline, we expect to guide the care of outpatients with COVID-19 in Brazil, highlighting the existing uncertainties, especially regarding the ineffectiveness or lack of documented benefit of most of the evaluated drugs. In addition to the evidence available in the scientific literature, the recommendations considered aspects relevant to the Brazilian context, such as the availability of drugs, the acceptability of interventions by the population and by clinicians, and the associated costs. Furthermore, most of the recommendations are currently in line with the therapeutic approaches recommended by the main international organizations and societies, such as the WHO, the NIH, and the IDSA.1,10,12,24
This document is a position statement supported by seven medical societies and was developed considering the need for comprehensive recommendations and the perspective of different medical specialties, given the weakness of available evidence and the relevance that should be given to the topic. Importantly, because of the large number of emerging therapies for COVID-19, these recommendations may need to be updated as new evidence appears, in particular randomized clinical trials of high methodological quality and new position statements issued by international societies and organizations.