Infectious keratitis is a sight-threatening condition that is usually an ocular emergency. The visual outcome depends on prompt and accurate clinical management as well as geographic and epidemiological awareness. We conducted a retrospective observational study to define the epidemiological and laboratory profile, as well as the clinical course of bacterial keratitis in a tertiary hospital in São Paulo over 21 years. Information about age, sex, predisposing factors, topical and surgical treatment, visual acuity, ulcers’ classification, bacterioscopy, culture, and antibiotic sensitivity tests were collected. This study included 160 patients. The mean age was 65.1 ± 18.4 years and risk factors were identified in 83.1 % of the patients. Empirical topical fortified cephalosporin with an aminoglycoside or fourth-generation fluoroquinolone was curative for 66.2 % of the cases. The mean treatment duration was 22.5 ± 9 days. The mean variation of visual acuity was -0.25 logMAR, p < 0.001. Culture revealed 64 % of Gram-positive bacteria. All Gram-positive bacteria were sensitive to cephalothin, vancomycin, and quinolones. All Gram-negative bacteria were sensitive to gentamicin, tobramycin, amikacin, and ciprofloxacin. These findings reinforce the importance of prompt empirical treatment of severe corneal ulcers with a fortified cephalosporin and aminoglycoside or a fourth-generation fluoroquinolone as there are equally effective. Collected data was insufficient to evaluate resistance of ocular infections over time in this population.
Infectious keratitis is a sight-threatening condition that is usually an ocular emergency.1 Approximately 2 million new cases of monocular blindness per year may be attributed to trauma or corneal ulcers worldwide.2 In the United States, the total cost for the treatment of keratitis was $174.9 million in 2010.3 Infectious keratitis is an inflammation and tissue destruction of the cornea associated with microbial proliferation of bacteria, fungi, viruses, or parasites.4 Sixty-five to ninety percent of infectious keratitis cases are caused by bacteria.5 Bacterial keratitis usually occurs in patients with predisposing factors that compromise the ocular surface defense mechanisms.6 Ocular risk factors include trauma, contact lens wear, corneal transplantation, corneal scarring disease, bullous keratopathy, meibomitis, lid abnormalities, herpetic keratitis.7 Systemic risk factors include systemic immunosuppression, either secondary to diseases or immunosuppressive agents, such as diabetes mellitus, and chronic alcoholism, encephalopathy, or coma.8,9 The visual outcome depends on prompt and accurate clinical management as well as geographic and epidemiological awareness.10 Traditionally, if an infection is suspected, corneal scraping smears and cultures should be performed,11 followed by the initiation of empiric, broad-spectrum, intensive therapy with antimicrobial agents and monitoring of the clinical response.12 The treatment includes two topical antibiotics (e.g., cephalosporin and aminoglycosides) at higher concentrations (fortified) than usually prescribed13 or a fourth-generation fluoroquinolone (moxifloxacin, gatifloxacin) as monotherapy.14 Community-acquired infections can usually be cured with only empirical treatment.15 However, if no clinical response is noticed, the ophthalmologist changes the treatment plan in accordance with the microbiological results. The widespread use of antibiotics worldwide can lead to the development of bacterial resistance to commonly used medications. Therefore, it is important to constantly supervise epidemiological data to update local treatment algorithms for infectious keratitis. Microbial spectrum and antibiotic sensitivity vary according to the geographical location,16 characteristics of the population,8 and changes brought about with time.17 The purpose of this study was to maintain epidemiological vigilance on the prevalence and clinical outcomes of bacterial keratitis in a tertiary-care hospital in the city of São Paulo, Brazil over a period of 21 years.
Material and methodsThis was a single-center, retrospective, observational, prognostic study. This study conformed to the provisions of the Declaration of Helsinki and was approved by the Ethics Committee of Hospital do Servidor Público Estadual de São Paulo (HSPE-SP) under the registration number CAAE 86296218.0.0000.5463.
Inclusion criteriaThis study included all patients treated for infectious keratitis who underwent corneal scrapings in the Cornea and External Disease Department of HSPE-SP between July 1997 and July 2018. HSPE-SP is the referral hospital for 1.2 million public employees from 163 cities of the State of São Paulo and covers mainly an urban area with a humid subtropical climate.
Exclusion criteriaThe exclusion criteria were immune-related, viral, Acanthamoeba or fungal ulcers, endophthalmitis, blebitis, dacryocystitis, scleritis, uveitis, conjunctivitis, and endothelitis.
Data collectionWe collected information about age, sex, predisposing systemic and ocular factors, clinical classification of ulcers, empirical treatment, the need for systemic antibiotics or surgery, corticosteroids and anti-collagenase use, duration of treatment, initial and final visual acuity, bacterioscopy and culture results, and antibiotic sensitivity test results. Ocular trauma, contact lens wear, ocular scarring disease, corneal transplant, bullous keratopathy, meibomitis, lid abnormalities, and herpetic keratitis were considered ocular risk factors. Systemic risk factors included systemic immunosuppression, either secondary to diseases or immunosuppressive agents, such as diabetes mellitus or individuals affected by human immunodeficiency viruses, and chronic alcoholism, drug use, rheumatoid arthritis, neoplasia, atopy, encephalopathy, or coma.
Ulcers’ classificationThe ulcers were classified as severe if they had a central or paracentral location, a diameter of 4 mm or more, involved more than one-third of the corneal thickness, and/or presented with a hypopyon. Moderate ulcers were peripheral, measured between 2 and 4 mm, affected only the anterior third of stromal depth, and had no hypopyon. Mild ulcers were < 2 mm in diameter.
Treatment optionsAt our institution, in cases of moderate and severe corneal ulcers, we scraped and cultured a sample and administered a topical combination of cephalothin or cefazolin (50 mg/mL) and gentamicin or tobramycin (14 mg/mL) every hour or prescribed a fourth-generation fluoroquinolone (moxifloxacin, gatifloxacin) as monotherapy. Systemic therapy was prescribed in severe cases with intraocular or scleral involvement, perforation, or gonococcal etiology. Fluorometholone 0,1 % ophthalmic suspension was prescribed after corneal epithelialization in selected cases: severe ulcers with intense inflammation, 2 to 3 days after initiation of broad-spectrum antibiotics with a positive clinical response but the absence of fungal or Pseudomonas suspicion. In cases of corneal melting and unfavorable progress, an oral anti collagenase, such as doxycycline 100 mg b.i.d., was prescribed.
Treatment durationDuration of treatment was defined as the time elapsed between the initial therapy implemented at the hospital and the complete healing of the ulcer (cure). Infection was considered as cured if there were no more inflammatory signs or symptoms, pain, stromal infiltrate, or anterior chamber reaction. Visual acuity was evaluated using the Snellen chart at the first visit and after the cure. For statistical analysis, the data were converted to a logarithmic scale.
MicrobiologyCorneal samples from infectious keratitis cases at HSPE-SP were collected in a standardized manner as follows. After topical anesthesia, a modified Kimura spatula was used to directly inoculate the scraped material from the bottom and border of the ulcer onto Gram stain and Giemsa colorations and blood agar, chocolate agar, Sabouraud agar, and enriched thioglycolate medium cultures.
The identification and antimicrobial susceptibility testing were initially performed using panels NMIC/ID 121 and PMIC/ID 105 in the system BD-Phoenix (Becton Dickinson). Susceptibility to ciprofloxacin, ceftriaxone, cefepime, teicoplanin, sulfamethoxazole-trimethoprim, amikacin, gentamicin, erythromycin, clindamycin, and tetracycline was evaluated by a disk diffusion method as recommended by the Clinical Laboratory Standards Institute (CLSI M100–23) and Brazilian Health Regulatory Agency (Anvisa). The susceptibility to vancomycin was determined by the broth microdilution method. S. aureus ATCC 25923 and E. faecalis ATCC 29212 were tested as quality‐control strains and the susceptibility testing results were interpreted based on the CLSI M100-S23 criteria and Anvisa.18
Statistical analysisData were analyzed using IBM Statistical Package for the Social Sciences (SPSS) 21.0 version. Numerical data were presented as mean ± Pattern Deviation (PD) or median and interquartile range, wherever applicable. Shapiro-Wilk and Kolmogorov-Smirnov were used to evaluate whether variables were normally distributed. For normal distribution data, t-Student test was applied. When variables were not normally distributed, Mann-Whitney test was chosen. The comparison of the mean of the quantitative variables between the categories of polytomous qualitative variables were not normally distributed and the Kruskal-Wallis H test was applied. The Wilcoxon test was used to compare two paired numerical data and X2 to compare categorical data. The alpha error was set at 5 %.
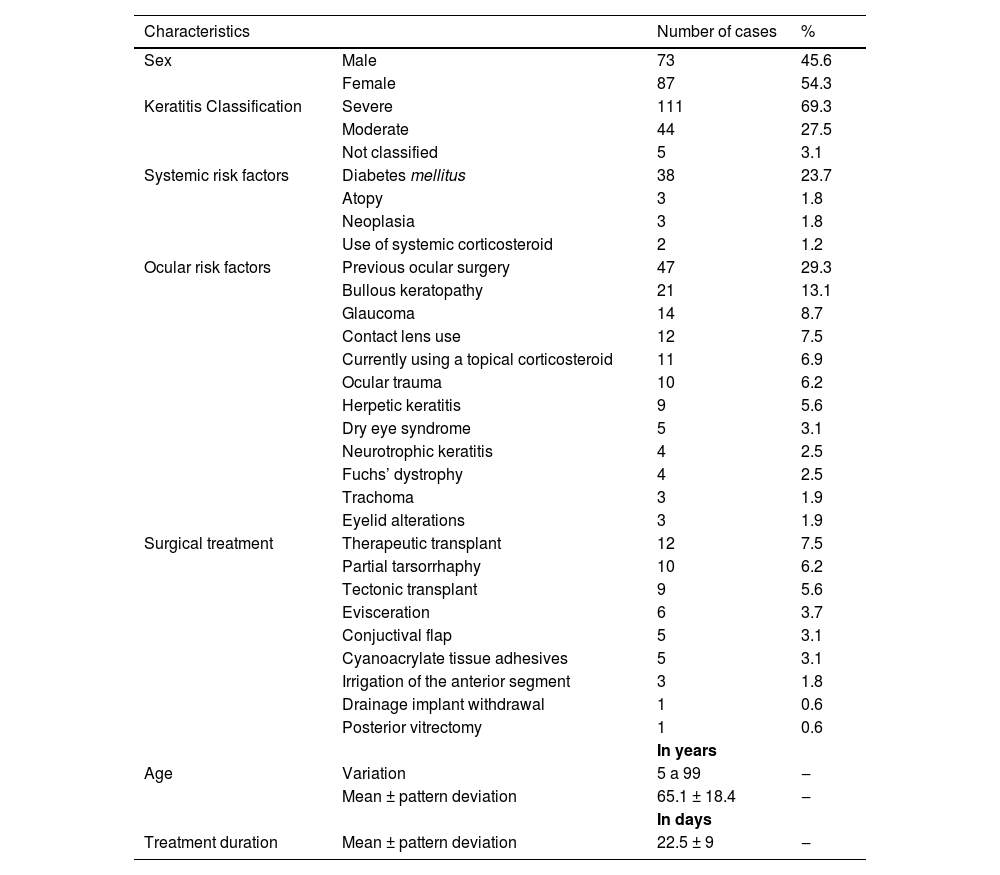
ResultsEpidemiological characteristicsFrom July 1997 to July 2018, 160 cases of infectious keratitis were reported. Table 1 describes the epidemiological characteristics of the study population. The mean age was 65.1 ± 1 8.4 years. Risk factors were identified in 83,1 % of the population of the study (Table 1).
Characteristics of patients and treatment for bacterial keratitis in Corneal and external diseases department in Hospital do Servidor Público Estadual de São Paulo (HSPE-SP) from July 1997 to July 2018.
Topical eye drops were used as initial empirical treatment in 159 patients (99.4 %): 105 (66 %) received fortified antibiotics (cephalothin or cefazolin 50 mg/mL and gentamicin or tobramycin 14 mg/mL) and 54 (33.9 %) were treated with only a fourth-generation quinolone. Data were unavailable for one patient. Surgical treatment was later performed in 40 patients (25 %) using the modalities shown in Table 1. Three patients (1.8 %) underwent more than one surgical treatment.
Clinical responseOne hundred and six patients (66.2 %) were cured with initial therapy. Forty-five patients (28.1 %) did not improve clinically and required a change of therapy: 26 (57.7 %) were using the fortified combination and 19 (42.2 %) were on quinolone. Data were unavailable for the nine remaining patients. No statistically significant difference was found between treatment with fortified combination versus fourth generation quinolone and clinical response (p = 0.16). Ten cases (20.8 %) out of the 45 patients with clinical deterioration had negative bacterioscopy and culture results and were subsequently treated with vancomycin (25 mg/mL) and amikacin (25 mg/mL). Three of those ten patients (30 %) treated with vancomycin and amikacin had infection resolved with topical treatment only. Seven of them (70 %) had to be submitted to corneal transplantation, and posteriorly three patients had to be eviscerated. In 19 cases (42.2 %), the etiological agent was isolated in the culture and guided the choice of medication, those patients achieved infection resolution with topical treatment. In 16 cases (35.5 %), other treatments were used: patients initially using quinolone started cephalothin and gentamicin, those already using a fortified combination initiated individual different treatments: moxifloxacin, gatifloxacin, acyclovir, ceftazidime, azithromycin, amphotericin B 0.15 %. Clinical evolution varied widely, but four eyes were eviscerated.
Adjuvant therapyIntravitreal injection of vancomycin with ceftazidime was administered to six patients (3.7 %). Oral antimicrobial therapy was used as adjuvant treatment in five patients (3.1 %): amoxicillin in two patients (1.2 %), and ciprofloxacin in three patients (1.8 %). Three patients (1.8 %) were treated with an intravenous antimicrobial agent, two (1.2 %) patients used ciprofloxacin and one (0.6 %) patient was advised vancomycin with ceftriaxone. Subconjunctival vancomycin injection was administered to three patients (1.8 %). Nine patients (5.6 %) evolved to endophthalmitis. A total of six eyes (3.7 %) were eviscerated, five of which (3.1 %) were previously amaurotic.
Seventy-five patients (46.8 %) were treated with topical corticosteroid and/or an oral anticollagenolytic: 8 patients (5 %) used both these medications, 41 patients (25.6 %) used only topical steroids, and 26 patients (16.2 %) used oral doxycycline.
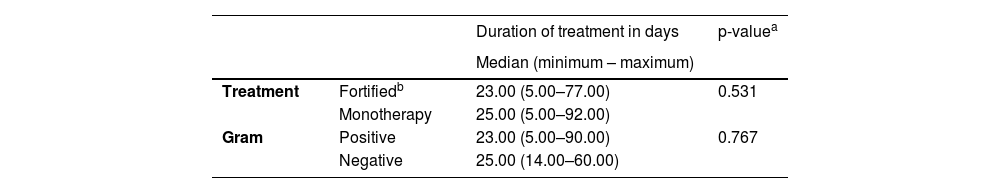
Treatment durationThe median time between symptom onset and treatment initiation was 5 (3‒9.5) days, varying from 1 to 60 days for 55 patients. Nineteen patients (34.5 %) waited for 1 to 3 days, 20 patients (36.4 %) waited for 4 to 7 days, and 16 patients (29.1 %) waited for more than 7 days before initiation of treatment. The mean duration of treatment was 22.5 ± 9 days. We compared duration of treatment of patients using quinolone versus fortified antibiotic (Table 2) and no statistically significant difference was found (p = 0.531). We also compared duration of treatment for Gram-positive versus Gram-negative bacteria (Table 2) and no statistically significant difference was found (p = 0.767).
Treatment duration comparing antibiotics and Gram classification of bacterial keratitis treated in Cornea and external disease department in Hospital do Servidor Público Estadual de São Paulo (HSPE-SP) from July 1997 to July 2018.
A comparison between pre- and post-treatment visual acuity was made for 76 patients. Information about visual acuity was unavailable for the remaining patients. The mean vision improvement was of two lines and two letters, or a mean variation of −0.25 logMAR (z = 3.36; p < 0.001). Thirty-eight patients (50 %) had the same vision, 28 (36.8 %) had improved visual acuity, and 10 (13.1 %) had worsened vision despite treatment. Eighteen eyes (23.7 %) were previously amaurotic.
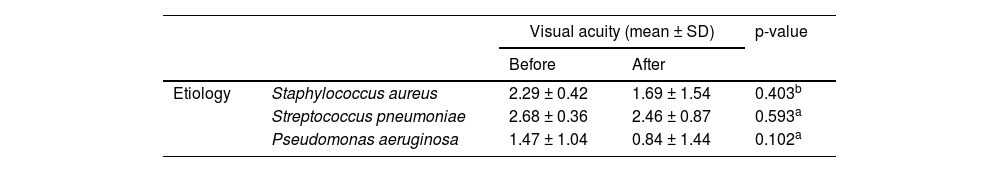
Pre- and post-treatment visual acuity for S. aureus, S. pneumoniae and P. aeruginosa were compared to search for a correlation between the etiology and visual prognosis, but no statistically significant difference was found (Table 3).
Visual acuity before and after treatment of the most common etiological agents of bacterial keratitis treated in Cornea and external disease department in Hospital do Servidor Público Estadual de São Paulo (HSPE-SP) from July 1997 to July 2018.
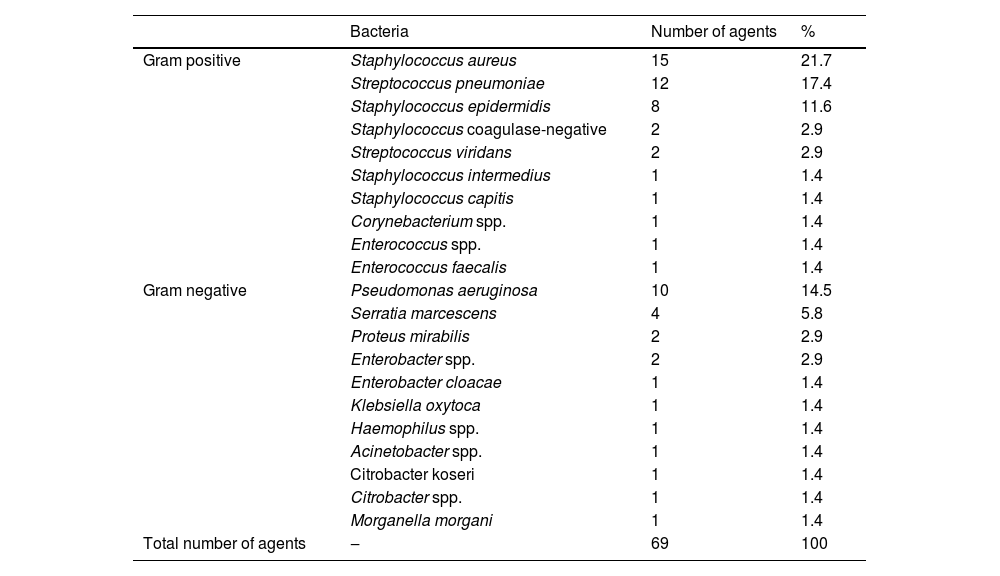
The culture was positive in 69 case (43.1 %). Gram-positive bacteria were the most common isolate (60.3 %): Staphylococcus spp. (37 %) was the first one in frequency followed by Streptococcus spp. (19.1 %). All isolated microorganisms are discriminated in Table 4. More than one specimen was identified in five samples (7.1 %).
Microorganisms isolated from the culture in patients with bacterial keratitis treated in Cornea and external disease department in Hospital do Servidor Público Estadual de São Paulo (HSPE-SP) from July 1997 to July 2018.
Gram-positive bacteria were less susceptible to the tested antibiotics than Gram-negative bacteria. Only six Gram-positive specimens (13.6 %) were sensitive to all the tested antibiotics. All microorganisms were sensitive to cephalothin and vancomycin, which are frequently used as empirical therapies. Resistance to oxacillin and penicillin was observed in seven cases (15.9 %). All Gram-positive bacteria were sensitive to all tested quinolones, except for a Staphylococcus intermedius strain resistant to moxifloxacin.
Gram-negative bacteria were sensitive to all the tested antibiotics, except for the following cases of resistance: a Serratia marcescens resistant to ceftriaxone and nitrofurantoin; a Citrobacter spp. resistant to cephalothin, and cefoxitin; Enterobacter resistant to amoxicillin-clavulanate, and cefuroxime; a Morganella morganii resistant to amoxicillin-clavulanate, and sulfamethoxazole-trimethoprim.
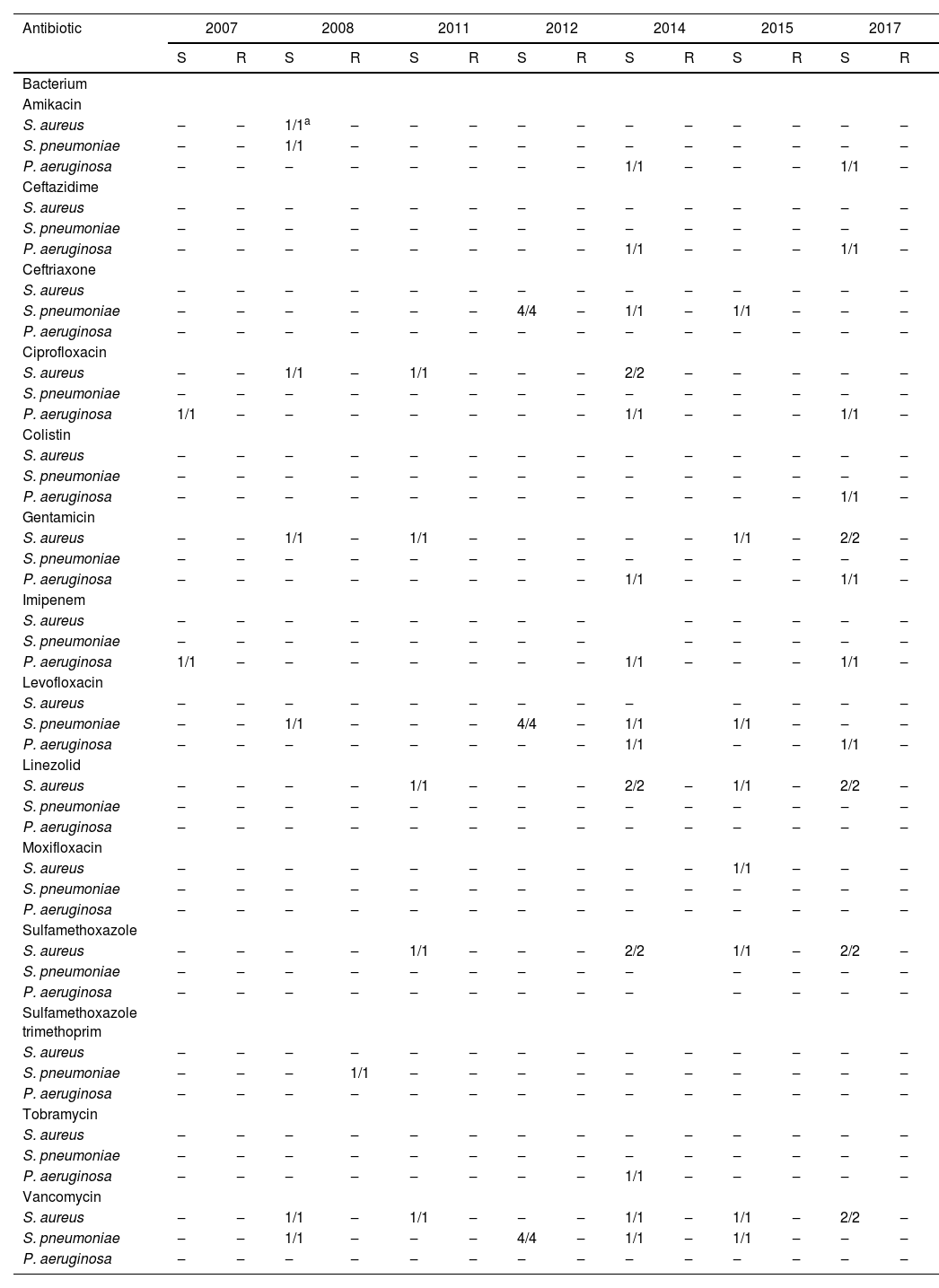
Data were analyzed in order to establish whether there was an increase in antibiotic resistance for S. aureus, S. pneumoniae and P. aeruginosa over time. No statistically significant difference was found. Resistance over time of tested antibiotics for S. aureus, S. pneumoniae e P. aeruginosa is shown in Table 5.
Antibiotic sensitivity test for most common agents isolated from patients with bacterial keratitis treated in Cornea and external disease department in Hospital do Servidor Público Estadual de São Paulo (HSPE-SP) from July 1997 to July 2018.
| Antibiotic | 2007 | 2008 | 2011 | 2012 | 2014 | 2015 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | S | R | S | R | S | R | S | R | |
| Bacterium | ||||||||||||||
| Amikacin | ||||||||||||||
| S. aureus | ‒ | ‒ | 1/1a | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 1/1 | ‒ |
| Ceftazidime | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 1/1 | ‒ |
| Ceftriaxone | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 4/4 | ‒ | 1/1 | ‒ | 1/1 | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Ciprofloxacin | ||||||||||||||
| S. aureus | ‒ | ‒ | 1/1 | ‒ | 1/1 | ‒ | ‒ | ‒ | 2/2 | ‒ | ‒ | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | 1/1 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 1/1 | ‒ |
| Colistin | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ |
| Gentamicin | ||||||||||||||
| S. aureus | ‒ | ‒ | 1/1 | ‒ | 1/1 | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | 2/2 | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 1/1 | ‒ |
| Imipenem | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | |
| P. aeruginosa | 1/1 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 1/1 | ‒ |
| Levofloxacin | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | |
| S. pneumoniae | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 4/4 | ‒ | 1/1 | 1/1 | ‒ | ‒ | ‒ | |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | 1/1 | ‒ | |
| Linezolid | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 2/2 | ‒ | 1/1 | ‒ | 2/2 | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Moxifloxacin | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Sulfamethoxazole | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 2/2 | 1/1 | ‒ | 2/2 | ‒ | |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | |
| Sulfamethoxazole trimethoprim | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Tobramycin | ||||||||||||||
| S. aureus | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| S. pneumoniae | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | ‒ | ‒ |
| Vancomycin | ||||||||||||||
| S. aureus | ‒ | ‒ | 1/1 | ‒ | 1/1 | ‒ | ‒ | ‒ | 1/1 | ‒ | 1/1 | ‒ | 2/2 | ‒ |
| S. pneumoniae | ‒ | ‒ | 1/1 | ‒ | ‒ | ‒ | 4/4 | ‒ | 1/1 | ‒ | 1/1 | ‒ | ‒ | ‒ |
| P. aeruginosa | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
S, Sensitive; R, Resistant.
Geographic and epidemiological awareness is crucial for accurate clinical management of bacterial keratitis. Our study showed that patients with the mean age of 65 years, a female majority (54 %) having a severe ulcer (69 %), and a history of diabetes mellitus (23.7 %), most commonly presented with infectious keratitis. In other studies, the mean age was inferior to ours, ranging from 25 to 57 years.8,19-23 This difference might be explained by the fact that our hospital provides care mainly to the elderly. Distribution among genders varies considerably in other studies.8,19-21,23,24 Diabetes was found to be the most common systemic condition in another referral hospital in São Paulo21 and India.25
Ulcers treated in our hospital were classified as severe in 69.3 % of the patients. This may be a selection bias since mild ulcers might have been treated in primary care with good evolution and without the need to be referred to tertiary care. In a series conducted in Los Angeles, severe cases represented 50.6 %15 of the total population diagnosed with infectious keratitis. In Oman, they were reported to be 36.17 %.26
Ocular surgery was the most common predisposing factor: 22 patients (13.7 %) had been previously submitted to corneal transplants. Our hospital serves as a reference for corneal grafts and this result might be due to a selection bias. Corneal grafts act as a predisposing factor for infection because of exposed or loose sutures that hurt the epithelium and accumulate mucus, favoring microbial colonization.27 Graft failure may present with microcystic epithelial edema28 because of chronic use of corticosteroids,27 among other causes. Traditionally, in developing countries, trauma is the most common ocular risk factor, as it was reported to be present in 90 % of keratitis cases in an Indian study.29 In other studies, such as in Brazil, it was present in 40 %22 and 25 %21 of the patients, respectively.
Most cases in our study were treated with topical fortified antibiotics (66 %). This is in accordance with an international survey of 140 cornea specialists, which reported that 80 % of North Americans empirically treated severe keratitis with fortified eyedrops.30 A recent study that evaluated 16 randomized clinical trials showed no difference between this treatment and monotherapy with quinolone.31
The use of topical corticosteroids as adjunctive therapy remains controversial. In our study, it was used in 49 patients (30.6 %). Those who support it argue that its anti- inflammatory property could reduce corneal opacification, neovascularization, and melting.32 The opponents say it would delay reepithelization, worsen the infection, and increase the risk of perforation.33 In a retrospective study, patients treated with a high dose of steroids presented with better visual acuity after treatment than those who did not receive high dose steroids.34 However, a Cochrane analysis of four randomized clinical trials concluded there was no statistically significant difference in terms of final visual acuity between a group treated with antibiotics compared to a group treated with adjunctive corticosteroids.35
The mean duration of treatment was 22.5 ± 9 days. This is in accordance with other studies that showed a duration of 15 to 30 days required to cure infectious keratitis, ranging from 7 to 46 days.31
Six eyes (3.7 %) were eviscerated. Other series reported 0 % to 1.9 % evisceration.10,36-38 The median time from the beginning of symptoms until the initiation of treatment was 5 (3‒9.5) days, with 29.1 % of patients waiting more than 7 days to opt for hospital care. Other Brazilian series reported the following percentage of treatment delay of more than 7 days: 85 %39 and 41.43 %.22
Our culture sensitivity was 43.1 %, which is inferior to other studies conducted in Brazil (62.8 %24 and 65 %21), Taiwan (49.3 %),17 Toronto (57.3 %),19 Vancouver (75 %),23 and India (59.3 %).29
The most common etiology of keratitis in 11 countries from 1999 to 2016 was Gram-positive bacteria, especially Staphylococci spp. and Streptococci spp. Pseudomonas aeruginosa was the most frequent Gram-negative bacteria.40–43 This is in accordance with our study, in which 64 % of bacteria were Gram-positive, with the most common agent being S. aureus, and P. aeruginosa was the most common Gram-negative isolate.17
Recently, there has been a concern regarding recrudescent bacterial resistance. In vitro susceptibility vigilance in the United States reported that only 15.2 % of methicillin-resistant S. aureus was susceptible to fluoroquinolones and that trimethoprim was the only efficient antibiotic that was tested.44 In our sample, all specimens were susceptible to quinolones except for one strain of S. intermedius that was resistant to moxifloxacin.
In Spain, over 10 years, S. aureus resistance to methicillin and levofloxacin was classified as moderate.45 In Toronto, increasing resistance to erythromycin, ceftazidime, and piperacillin-tazobactam was identified.19 In Taiwan, an increasing susceptibility to oxacillin and clindamycin has been noted.17
The benefit of this study is the long period evaluated and the characterization of the clinical outcomes of bacterial keratitis treated in a referral tertiary hospital in Brazil. The study also had some limitations, especially due to this small sample of bacterial keratitis (160 cases), probably because of the afore-mentioned selection bias (only severe cases of ulcers are referred to our Corneal department). Furthermore, we also faced the information bias of a retrospective study with important pieces of information lacking from the patient`s records, especially ancient antibiotic sensitivity tests.
ConclusionThese findings reinforce the importance of prompt empirical treatment of severe corneal ulcers with a fortified cephalosporin and aminoglycoside or a fourth-generation fluoroquinolone as there are equally effective. Collected data were insufficient to evaluate resistance of ocular infections over time in Brazil.
FundingThis research has received no funding.
We would like to thank Editage (www.editage.com) for English language editing.