Hematopoietic progenitor cells from peripheral blood (HPCPB) are commonly used for autologous and allogenic transplants in patients with most various onco-hematological diseases, and despite the utilization of sterile techniques during collection and processing of these products, bacterial contamination can occur. This study aimed to investigate the microbial contamination of HPCPB products. Microbial cultures of 837 HPCPB products between the year 2000 and 2009 were retrospectively analyzed to determine the incidence of culture positivity and identify the main organisms that cause contamination. The microbiological studies were performed with an automated system (BacT/Alert® bioMérieux Corporate). Thirty-six (4.3%) of 837 microbial cultures were contaminated. Coagulase-negative Staphylococcus was the most frequent bacteria isolated from HPCPB products (20 [56%] of the 36 positive microbial cultures). Considering the 36 contaminated samples, 22 HPCPB products were infused and 14 discarded. Pre- and post-infusion antibiotic therapy of the patients transfused with contaminated products was established based on the isolated microorganism and its antibiogram. Microbial contamination rate of HPCPB products was low. Clinically significant outcomes after infusion of contaminated HPCPB products were not observed.
Hematopoietic progenitor cells from peripheral blood (HPCPB) are commonly used for autologous and allogenic transplants in patients with various onco-hematological diseases. The progenitor hematopoietic cells are capable of self-renewal and differentiation in all blood cells lineages. Bone marrow is the traditional source for obtaining HPCPB, collected by multiple punctures and aspirations of the posterior iliac crests. The aspirated material contains red blood cells, leukocytes, platelets, mast cells, plasma, and pluripotent hematopoietic progenitor cells. In recent years, the collection of HPCPB via apheresis has been increasingly used. The combination of high doses of chemotherapy with subsequent transplantation of these cells constitutes the standard treatment for many onco-hematological diseases.
Obtaining, processing, storing and transplantation of HPCPB involve many steps, which are normally performed in different environments and may result in microbial contamination. In fact, HPCPB manipulation during processing and pre- and post-cryopreservation are important sources of bacterial contamination of these cells.1 The donor may be the source of microbial contamination of HPCPB. Donors with asymptomatic bacteremia or who are recovering from a bacterial infection may develop episodes of transient bacteremia, which can lead to product contamination. In addition, HPCPB collected by apheresis often requires the insertion of central venous catheters (CVC). Infections associated with CVCs are an important source of transient bacteremia and a possible cause of HPCPB contamination.2
Thus, in order to ensure a final product appropriate for transplant, it is essential to adhere to a quality control policy. Such controls should include CD34+cell count, cell viability assessment, and microbiological monitoring.3
The main objective of this study was to investigate the incidence of positive microbial cultures for HPCPB products from donors attending a tertiary care hospital in the period from 2000 to 2009. In parallel, the major bacteria contaminating HPCPB products and the pre- and post-infusion antibiotic therapy for the contaminated cells were also described. In addition, the blood culture results after thawing the bag containing HPCPB, which were infused or discarded according to medical decision, were also analyzed (Table 1).
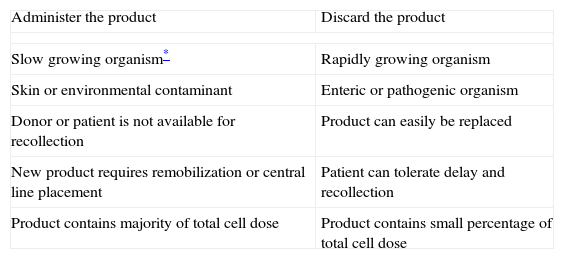
Management considerations of HPCPB products with positive microbial cultures.
| Administer the product | Discard the product |
| Slow growing organism* | Rapidly growing organism |
| Skin or environmental contaminant | Enteric or pathogenic organism |
| Donor or patient is not available for recollection | Product can easily be replaced |
| New product requires remobilization or central line placement | Patient can tolerate delay and recollection |
| Product contains majority of total cell dose | Product contains small percentage of total cell dose |
HPCPB, hematopoietic progenitor cells from peripheral blood.
Microbial cultures of 837 HPCPB products of donors attending a tertiary care hospital located in southern Brazil from 2000 to 2009 were retrospectively analyzed to determine the incidence of microbial culture positivity and identification of the main organisms causing contamination. In addition, the charts of the donors with positive HPCPB microbial cultures were reviewed.
For the sterility control of the HPCPB products, after the cryopreservation process and before freezing, 3mL samples of the product were inoculated in pediatric blood culture bottles with 20mL of activated charcoal (BacT/Alert® bioMérieux Corporate–Durham, USA). In addition, after blood bag thawing, samples were collected for microbial cultures at the moment of the infusion. Such action serves to verify a possible contamination at the time of water-bath defrosting. Cultures were sent to the microbiology department, where they were incubated for five days. When positive, microscopy and bacterial isolation were performed and identified through standard biochemical tests.
Data were organized and analyzed using the Microsoft Excel 2007® software, according to the distribution of frequency.
Microbiological surveys were performed with automated BacT/Alert® at 36°C. The products were added into a class I laminar-flow cabinet with HEPA filters.
The study was approved by the local ethics committee, which is accredited by the National Committee of Ethics in Research of the National Health Department and the Office for Human Research Protection (OHRP) of the United States.
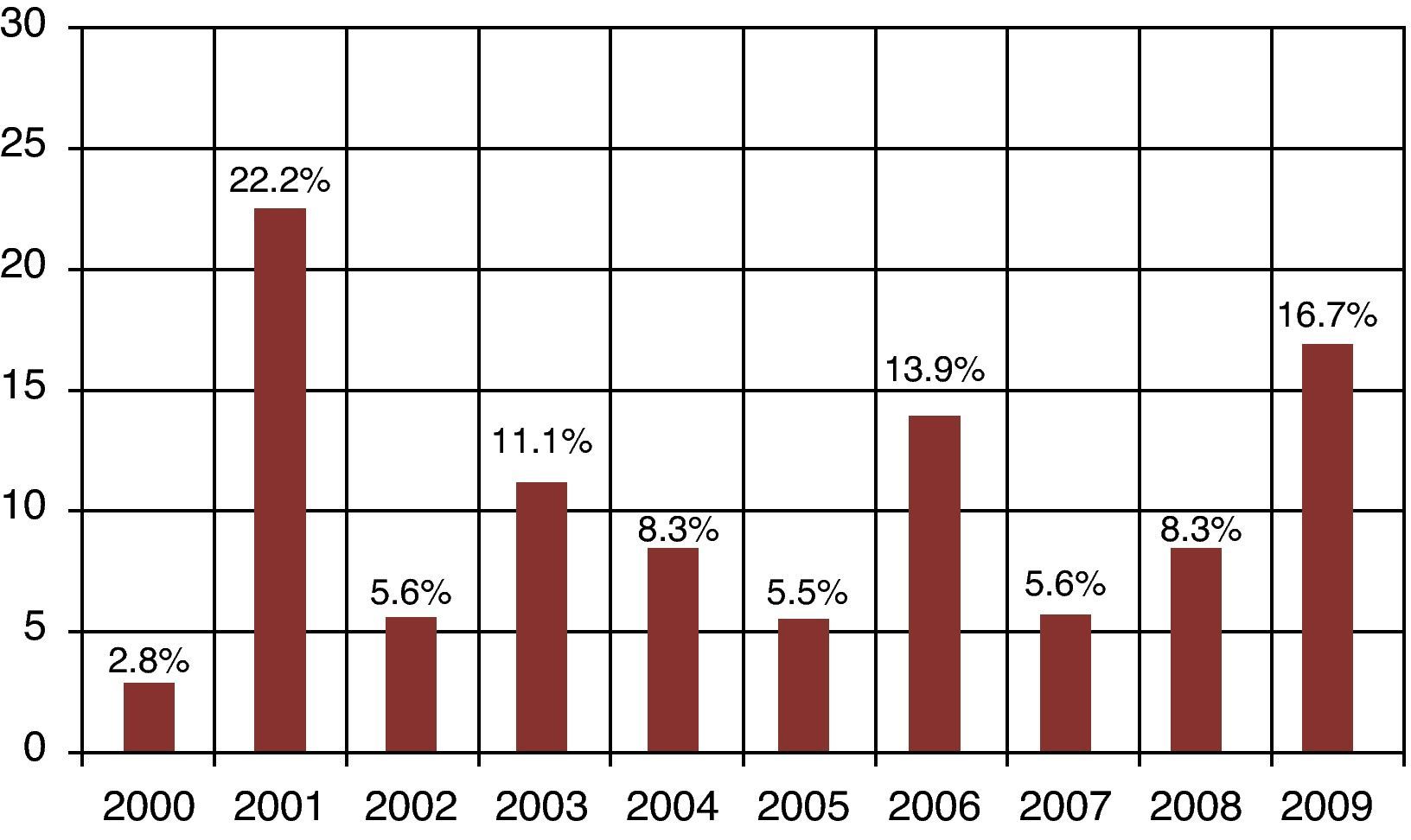
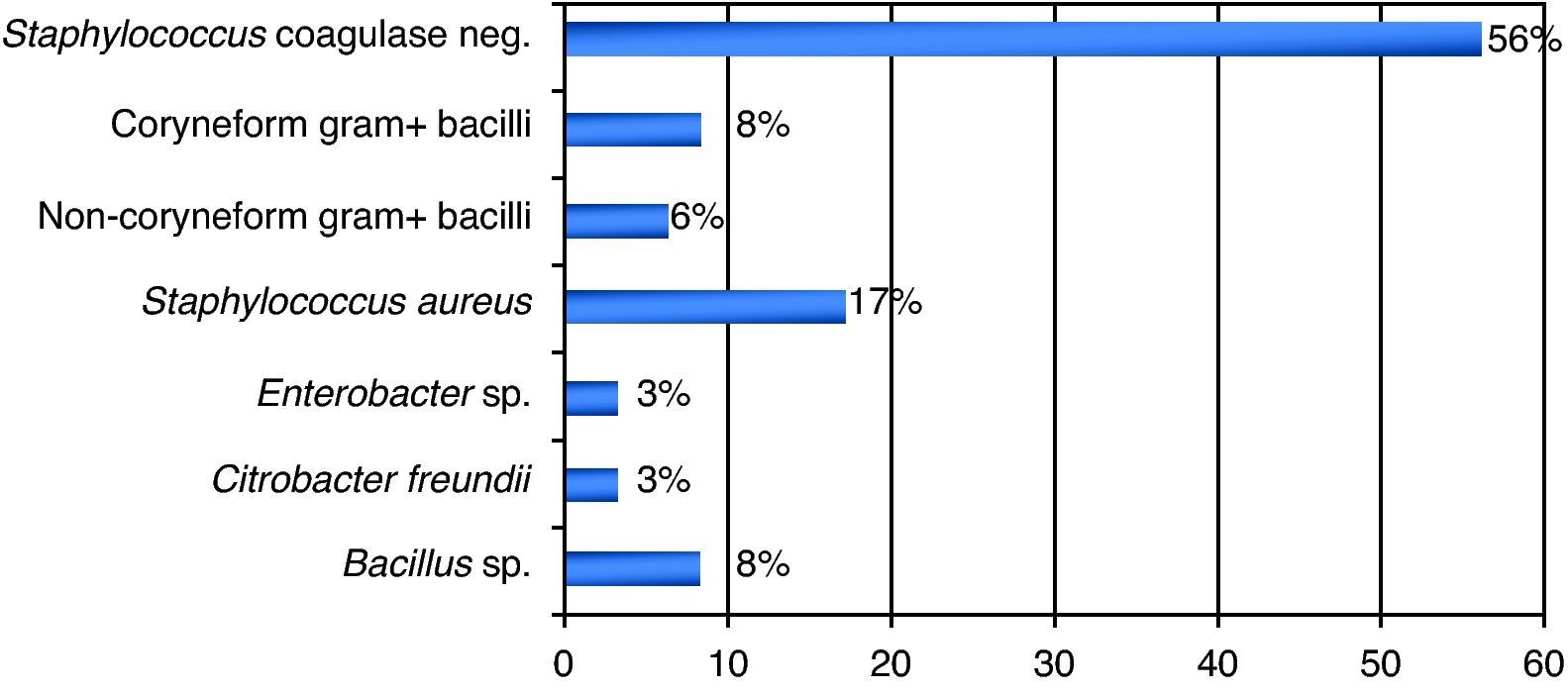
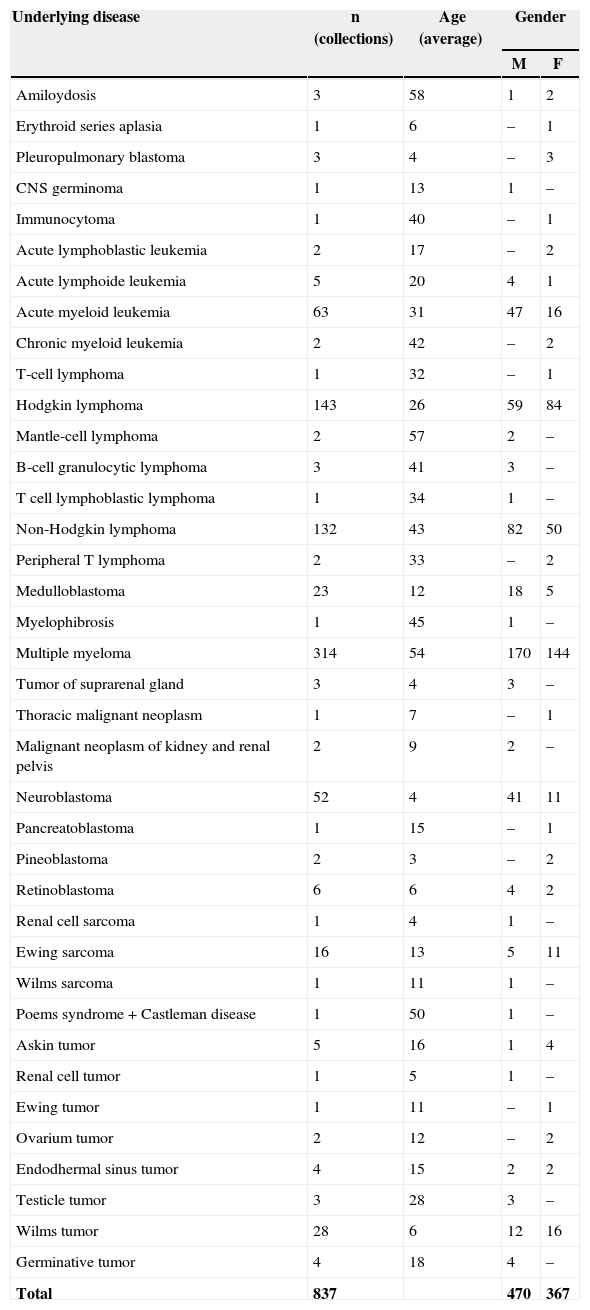
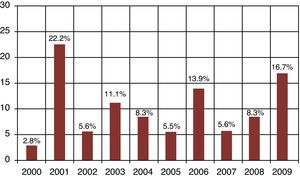
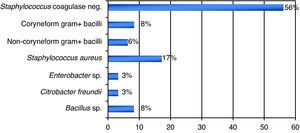
ResultsA total of 837 HPCPB collections and microbial cultures were performed at the hemotherapy section from 2000 to 2009. The average volume drawn and time for collection and processing were 255mL and 206minutes respectively. The underlying diseases and the main characteristics of the patients that received HPCPB products are presented in Table 2. The main underlying diseases included multiple myeloma (n=314), followed by Hodgkin lymphoma (n=143), non-Hodgkin lymphoma (n=132), acute myeloid leukemia (n=63), neuroblastoma (n=52), Wilms tumor (n=28), meduloblastoma (n=23), and Ewing sarcoma (n=16).Thirty-six of the 837 collected samples (4.3%) yielded positive cultures for bacteria. Fig. 1 presents the annual contamination rate of the HPCPB products from 2000 to 2009. As shown in Fig. 2, the most frequently isolated organism was coagulase-negative Staphylococcus (56%), followed by Staphylococcus aureus (17%), Bacillus sp. (8%), coryneform Gram-positive bacilli (8%), non-coryneform Gram-positive bacilli (6%), Enterobacter sp. (3%), and Citrobacter freundii (3%). Coagulase-negative Staphylococcus isolates were 100% resistant to beta-lactams antibiotics including oxacillin.
Profile of underlying diseases and HPCPB collections performed at the Hemotherapy Section from 2000-2009.
| Underlying disease | n (collections) | Age (average) | Gender | |
|---|---|---|---|---|
| M | F | |||
| Amiloydosis | 3 | 58 | 1 | 2 |
| Erythroid series aplasia | 1 | 6 | – | 1 |
| Pleuropulmonary blastoma | 3 | 4 | – | 3 |
| CNS germinoma | 1 | 13 | 1 | – |
| Immunocytoma | 1 | 40 | – | 1 |
| Acute lymphoblastic leukemia | 2 | 17 | – | 2 |
| Acute lymphoide leukemia | 5 | 20 | 4 | 1 |
| Acute myeloid leukemia | 63 | 31 | 47 | 16 |
| Chronic myeloid leukemia | 2 | 42 | – | 2 |
| T-cell lymphoma | 1 | 32 | – | 1 |
| Hodgkin lymphoma | 143 | 26 | 59 | 84 |
| Mantle-cell lymphoma | 2 | 57 | 2 | – |
| B-cell granulocytic lymphoma | 3 | 41 | 3 | – |
| T cell lymphoblastic lymphoma | 1 | 34 | 1 | – |
| Non-Hodgkin lymphoma | 132 | 43 | 82 | 50 |
| Peripheral T lymphoma | 2 | 33 | – | 2 |
| Medulloblastoma | 23 | 12 | 18 | 5 |
| Myelophibrosis | 1 | 45 | 1 | – |
| Multiple myeloma | 314 | 54 | 170 | 144 |
| Tumor of suprarenal gland | 3 | 4 | 3 | – |
| Thoracic malignant neoplasm | 1 | 7 | – | 1 |
| Malignant neoplasm of kidney and renal pelvis | 2 | 9 | 2 | – |
| Neuroblastoma | 52 | 4 | 41 | 11 |
| Pancreatoblastoma | 1 | 15 | – | 1 |
| Pineoblastoma | 2 | 3 | – | 2 |
| Retinoblastoma | 6 | 6 | 4 | 2 |
| Renal cell sarcoma | 1 | 4 | 1 | – |
| Ewing sarcoma | 16 | 13 | 5 | 11 |
| Wilms sarcoma | 1 | 11 | 1 | – |
| Poems syndrome+Castleman disease | 1 | 50 | 1 | – |
| Askin tumor | 5 | 16 | 1 | 4 |
| Renal cell tumor | 1 | 5 | 1 | – |
| Ewing tumor | 1 | 11 | – | 1 |
| Ovarium tumor | 2 | 12 | – | 2 |
| Endodhermal sinus tumor | 4 | 15 | 2 | 2 |
| Testicle tumor | 3 | 28 | 3 | – |
| Wilms tumor | 28 | 6 | 12 | 16 |
| Germinative tumor | 4 | 18 | 4 | – |
| Total | 837 | 470 | 367 | |
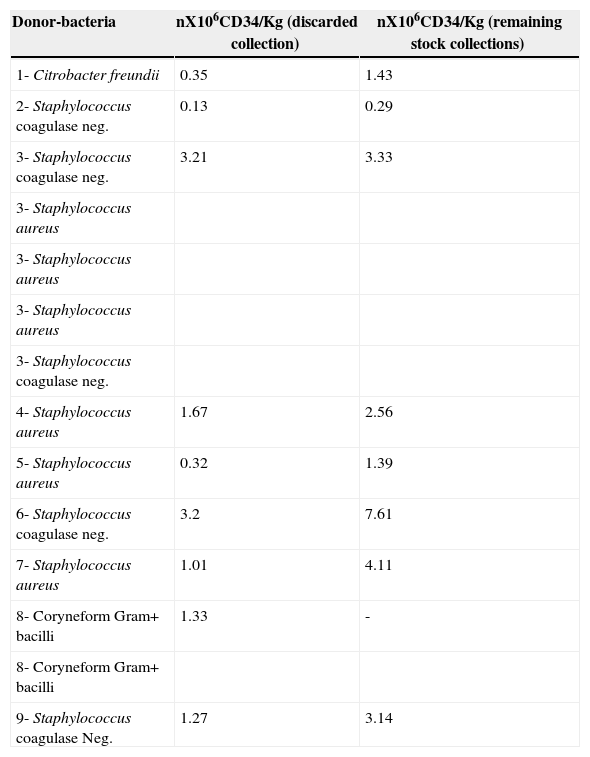
Twenty-two of the 36 HPCPB products with positive microbial cultures were infused. and 14 were discarded based on the medical staff's decision. Considering that HPCPB products contamination has an impact in reducing the number of CD34+ cells, and therefore reducing hematopoietic engraftment after peripheral blood stem cell transplantation, Table 3 presents the CD34+ counts of the discarded contaminated HPCPB collections and the remaining stock collections for each patient.
CD34+ count of contaminated and remaining stock HPCPB collections.*
| Donor-bacteria | nX106CD34/Kg (discarded collection) | nX106CD34/Kg (remaining stock collections) |
|---|---|---|
| 1- Citrobacter freundii | 0.35 | 1.43 |
| 2- Staphylococcus coagulase neg. | 0.13 | 0.29 |
| 3- Staphylococcus coagulase neg. | 3.21 | 3.33 |
| 3- Staphylococcus aureus | ||
| 3- Staphylococcus aureus | ||
| 3- Staphylococcus aureus | ||
| 3- Staphylococcus coagulase neg. | ||
| 4- Staphylococcus aureus | 1.67 | 2.56 |
| 5- Staphylococcus aureus | 0.32 | 1.39 |
| 6- Staphylococcus coagulase neg. | 3.2 | 7.61 |
| 7- Staphylococcus aureus | 1.01 | 4.11 |
| 8- Coryneform Gram+ bacilli | 1.33 | - |
| 8- Coryneform Gram+ bacilli | ||
| 9- Staphylococcus coagulase Neg. | 1.27 | 3.14 |
HPCPB, hematopoietic progenitor cells from peripheral blood.
HPCPB, hematopoietic progenitor cells from peripheral blood; M, masculine; F, feminine; CNS, central nervous system.
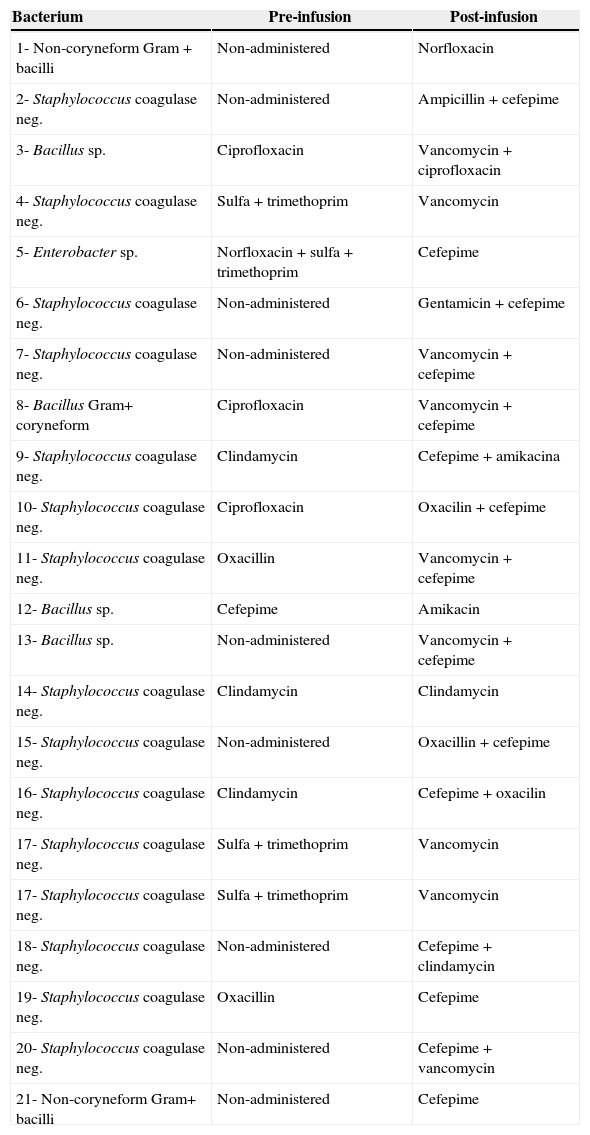
Table 4 presents pre-infusion and post-infusion antibiotic therapy of the patients transfused with contaminated products. Although nine of the 22 infusions had not received antimicrobial therapy prior to the infusion of HPCPB products, all of the patients received such therapy during or after the HPCPB infusion.
Antibiotic therapy of pre- and post-infusion of contaminated HPCPB.*
| Bacterium | Pre-infusion | Post-infusion |
|---|---|---|
| 1- Non-coryneform Gram+bacilli | Non-administered | Norfloxacin |
| 2- Staphylococcus coagulase neg. | Non-administered | Ampicillin+cefepime |
| 3- Bacillus sp. | Ciprofloxacin | Vancomycin+ciprofloxacin |
| 4- Staphylococcus coagulase neg. | Sulfa+trimethoprim | Vancomycin |
| 5- Enterobacter sp. | Norfloxacin+sulfa+trimethoprim | Cefepime |
| 6- Staphylococcus coagulase neg. | Non-administered | Gentamicin+cefepime |
| 7- Staphylococcus coagulase neg. | Non-administered | Vancomycin+cefepime |
| 8- Bacillus Gram+ coryneform | Ciprofloxacin | Vancomycin+cefepime |
| 9- Staphylococcus coagulase neg. | Clindamycin | Cefepime+amikacina |
| 10- Staphylococcus coagulase neg. | Ciprofloxacin | Oxacilin+cefepime |
| 11- Staphylococcus coagulase neg. | Oxacillin | Vancomycin+cefepime |
| 12- Bacillus sp. | Cefepime | Amikacin |
| 13- Bacillus sp. | Non-administered | Vancomycin+cefepime |
| 14- Staphylococcus coagulase neg. | Clindamycin | Clindamycin |
| 15- Staphylococcus coagulase neg. | Non-administered | Oxacillin+cefepime |
| 16- Staphylococcus coagulase neg. | Clindamycin | Cefepime+oxacilin |
| 17- Staphylococcus coagulase neg. | Sulfa+trimethoprim | Vancomycin |
| 17- Staphylococcus coagulase neg. | Sulfa+trimethoprim | Vancomycin |
| 18- Staphylococcus coagulase neg. | Non-administered | Cefepime+clindamycin |
| 19- Staphylococcus coagulase neg. | Oxacillin | Cefepime |
| 20- Staphylococcus coagulase neg. | Non-administered | Cefepime+vancomycin |
| 21- Non-coryneform Gram+ bacilli | Non-administered | Cefepime |
HPCPB, hematopoietic progenitor cells from peripheral blood.
Twelve (55%) of the 22 contaminated HPCPB products presented positive microbial cultures after the freeze-thaw process, whereas in the remaining products (45%) the cultures were negative.
DiscussionSimilar to the present results, previous studies have reported microbial contamination rates varying from 1.6% a 4.5%.4–8 The incidence of microbial contamination of HPCPB products in those studies varied according to the source of the cells. Kamble et al.5 have shown contamination in four of the 26 collections (15%) from core blood, eight of 177 (4.5%) from bone marrow, and 21 of 532 (3.9%) from peripheral blood. Coagulase-negative Staphylococcus was the organism predominantly isolated in this study, with 20 (56%) of the 36 positive microbial cultures. Most of the previous studies also identified the coagulase-negative Staphylococcus and other bacteria that often colonize the skin and are water contaminants.9–12 The potential contamination sources of HPCPB products include reagents, venous access-catheters, aseptic failure, cell processing, bag disruption, equipment used for water-bath, incubators, and centrifuges.13–17
Even though contaminated HPCPB products are often discarded, 22 of the 36 contaminated collections were infused. Authors have reported success achieved after the infusion of the contaminated HPCPB products, with few clinical consequences.9,18 In fact, contaminated HPCB products should not be automatically discarded because, when administered with specific precautions, they do not have either adverse effects or significant sequelae. As shown in Table 1, specific measurements should be considered when deciding to discard or administer the product.19
CD34+ cell dose is correlated with both early engraftment kinetics and late peripheral blood counts. A threshold effect between rapid and slow engraftment occurred at 5×106 CD34+ cells/kg. In addition, hemoglobin and platelets were significantly higher at 180, 360, and 540 days after transplantation for those patients who received >5×106 CD34+ cells/kg. Therefore, for autologous transplantations, a dose higher than CD34+ cell appears to be correlated with improved long-term hematopoiesis. In this study, most of the contaminated collections presented low CD34+ cell dose. Despite contamination, some frozen stocks of those collections from these patients presented high CD34+ cell dose, which could be successfully used in further HCPBC transplants.
Contamination of HPCPB products with clinically significant adverse outcome occurs especially with potentially pathogenic bacteria, but is rare, with an incidence of 0.3% of notified cases.5 Klein et al.20 have reported a patient that died due to multi-organ-system failure, after having received HPCPB product contaminated with Burkholderia cepacia, even though the infusion was initiated with proper antimicrobial therapy. Moreover, contaminated HPCPB products with methicillin-resistant Staphylococcus aureus have been shown to cause severely disseminated infection in patients.5 Interestingly, bacterial contamination of those products does not affect the patients’ transplant kinetics. It was previously demonstrated by Schwella et al.12 that there was no significant differences in hematopoietic recovery time, duration of fever, and number of days of antimicrobial administration in patients who had received contaminated HPCPB products when compared with those who had received products free of contamination.12 In fact, previous studies have shown that most patients successfully receive an HPCPB product with prophylactic antibiotic therapy before infusion of the contaminated product based on the isolated organism, sensitivity to antimicrobial agents and urgency for the transplant.20 This decision varies in different centers. For instance, Kamble et al.5 have reported that prophylaxis is unnecessary, because most of the contaminations are caused by non-pathogenic bacteria and their infusions rarely cause bacteremia or septicemia. Interestingly, some patients infused with collections contaminated with coagulase-negative Staphylococcus that were treated with inappropriate prophylactic antibiotics such as oxacillin and ciprofloxacin did not present any unfavorable outcome.
In the present study approximately 50% of the HPCPB collections were found to persist contaminated after the freeze-thaw process. One of the reasons for this finding could be the contamination of HPCPB products with a low number of colony forming units of bacteria. In addition, cryoprotectors used in HPCPB products, such as the organosulfur compound dimethyl sulfoxide (DMSO), have potent bactericidal properties against Gram-positive and Gram-negative bacteria.19 Kipp et al.6 have shown that the CFUs of different bacteria diminished after the cryopreservation process. For instance, the CFU of Staphylococcus epidermidis, decreased approximately 13.7% after addition of DMSO. Moreover, the presence of active phagocytic cells in the frozen products could additionally eliminate existing bacteria.6 On the other hand, Gram-positive bacteria such as Staphylococcus sp. are known to survive after the cryopreservation process.5 Different studies have shown conflicting results regarding the survival of coagulase-negative Staphylococcus after the cryopreservation process.9,12,16
Data have shown that aseptic conditions impact bacterial contamination in areas where HPCPB products are handled and processed. There is a 5.2% decrease in HPCPB products contamination at a clean bench compared with 0.8% decrease at a bench in laboratory implementing good manufacturing practices with certified conditions.11 Thus, quality control and good practices of handling and conservation of reagents and equipment used in cell cryopreservation are essential to provide safer products for patients, with reduction of the probable contamination sources.21
In summary, our study has shown that the contamination rate of HPCPB products is overall low and it is usually caused by the normal skin microbiota, which could survive the cryopreservation process. No clinically significant outcomes were observed in patients transfused with contaminated HPCPB products. Continuous monitoring of HPCPB products is essential to assure the success of the transplantation.
Conflict of interestAll authors declare to have no conflict of interest.