It is debatable whether HIV-infected patients are at greater risk for hepatitis E virus (HEV) infection compared with healthy subjects. The reported anti-HEV seroprevalence among different groups in Bulgaria varied from 9.04% to 25.9%, but the information regarding the HIV population is still missing. The aim of the present study was to evaluate hepatitis E seroprevalence among HIV-infected patients in Bulgaria and to analyze demographic and immunological factors associated with HEV infection. Serum samples of 312 HIV-infected patients were analyzed retrospectively. Age, sex, residence and laboratory markers for HEV, HBV, HCV and HIV infection, and lymphocytes subpopulations were collected for all patients. None of the tested samples were positive for HEV RNA. HEV seroprevalence among HIV-infected patients was 10.9%. Males were more affected with the highest prevalence of positivity in the age group > 30 to ≤ 40 years. The documented HIV transmission routes in HIV/HEV co-infected group were heterosexual, homosexual, intravenous drug use (IDU), and vertical with predominace of the heterosexual route (z = 0.2; p = 0.804). There was a statistically significant trend of HIV mixed infection with routes of HIV transmission other than homosexual - heterosexual in HIV/HEV group and injection drug use in HIV/HBV/HCV co-infected group. The route of HIV transmission, in contexts of patients’ behavior, was associated with HEV prevalence among HIV-infected patients.
Hepatitis E (HEV) is an enterically transmitted quasi-enveloped virus, which spreads among animals and humans. The geographical distribution, host range and clinical presentation of the infection depend on the virus genotype. Currently, there are nine known HEV genotypes, but there may be more.1 HEV genotypes 1 and 2 are strictly anthroponotic with the ability to cause large water-born epidemics, affecting people from different age groups. The infection is mild to self-limited, but can cause fulminant hepatic failure and high mortality in pregnant women.2 HEV genotypes 3 and 4 are zoonotic and domestic pigs, wild boars, deers and rabbits are the main reservoirs for humans. Transmission to human is fecal-oral and occurs through feces, direct contact with infected animals and their offal, and consumption of contaminated meat products.3 In immunocompetent individuals the infection is asymptomatic and self-limited, but can progress to acute liver failure in elderly patients. Chronic HEV infection can occur in immunosuppressed patients, as solid organ transplant and HIV-infected patients.4 HEV infection can present with various extrahepatic manifestations - neurological, renal, cardiac, and hematological.5 It is debatable whether HIV-infected patients are at greater risk of HEV infection comparing with healthy subjects. A meta-analysis of studies in Europe estimated an HEV seroprevalence ranging from 0.6% to 52.5% and increasing with age, but unrelated to sex.6 The established HEV seroprevalence among HIV-infected individuals varies among countries and exceeds 40% in African countries, 20% to 10 % in European countries, and up to 10% in the Americas.7
The detection of specific HEV antibodies or/and HEV RNA in serum are essential tools for diagnosing acute or chronic HEV infection. At the same time, the duration of the anti-HEV response is still unknown. Statistically, it was estimated that after recovery from HEV infection this period can exceed 50 years and 30 years after vaccination.8 It is known that a low T cells (CD4+) count may delay or lack IgG seroconversion or may ensue anti-HEV IgG seroreversion.9 HIV infection is characterized with profound T-cell depletion in blood and tissues and immune exhaustion. The virus specific T-cells are essential for immunopathogenesis of acute and chronic viral hepatitis, as well for the development of the extrahepatic manifestations.10 A moderate to strong multispecific T-cell response is established in recovered anti-HEV IgG positive immunosuppressed patients after acute HEV infection. The detected memory T‐cell responses against HEV were much stronger than the T‐cell responses detectable during or after acute hepatitis B or C.11 Thereby, symptomatic HEV infections are characterized by the expansion of activated effector memory CD8 T-cells, which leads to T-cells exhaustion.12
Currently, there are no studies on the overall HEV prevalence in Bulgaria. Separate studies have been conducted, where the reported anti-HEV seroprevalence varied from 9.04%13 to 25.9 %14; among patients on hemodialysis, the anti-HEV seroprevalence of 14.7% was observed.15 In accordance with Ordinance No. 21 on the Procedure for Registration, Reporting and Control of Infectious Diseases, the acute form of viral hepatitis E is subject to mandatory registration and reporting since 2019. Newly HIV diagnosed cases started to be reported in 1986. Up to May 2021 there were 3571 registered cases in Bulgaria,16 and in 2019 alone 258 cases were reported with a rate of 3.7 per 100,000 population.17 The prevalence of anti-HEV among HIV-infected patients in Bulgaria is unknown. The aim of the present study was to evaluate the HEV seroprevalence among HIV-infected patients in Bulgaria and to analyze demographic and immunological factors associated with HEV infection.
MethodsStudy design and study populationThis was a retrospective cohort study designed to estimate the association between HEV seropositivity and demographical and immunological factors, among HIV-infected patients. This study cohort consisted of 312 confirmed HIV-infected persons, who were newly diagnosed or being followed-up in different specialized hospitals for inpatient and outpatient treatment of HIV under the jurisdiction of the Bulgarian Ministry of Health. Serological and molecular tests of HEV, HBV, and HCV, in addition to T lymphocytes subpopulations count were performed during routine screening of the patients. The study was approved by the Institutional Review Board/Institutional Ethics Committee (IRB 00006384) of the NCIPD with decision No2/2019. Written informed consent was waived because the samples drawn were part of HIV screening and follow-up care.
Group assignmentSerum samples of HIV-infected patients sent for viral hepatitis screening between December, 2019 and March, 2021, were included. The samples were sub-grouped as HIV/HEV group - positive for anti-HEV IgM and/or anti-HEV IgG and/or HEV RNA. Two control groups were 1) HIV-mono – negative for HBV, HCV and HEV; and 2) HIV/HBV/HCV – positive for HBV or/and HCV, but negative for anti-HEV.
Serological assaysAntibodies against HEV (anti-HEV IgM and IgG) were detected by ELISA (Euroimmun, Germany) in accordance with the manufacturer's instructions. The tested samples were considered reactive for anti-HEV IgM at a signal/cut-off ratio (S/CO) of 1.1 or greater. For anti-HEV IgG the results were interpreted quantitatively in [IU/ml] in accordance with the manufacturer's instructions, where the results ≥ 1.1 [IU/ml] were considered positive. Additional all studied samples were tested by ELISA for HBsAg (DiaPro, Italy), and for anti-HCV (DiaPro, Italy).
Quantification of the viral loadDetection and quantification of HEV RNA was performed by RealStar HEV RT-PCR kit 2.0 (Altona diagnostics, Germany) in accordance with the manufacturer's instructions. The minimal linear limit of quantitation of the kit was 10 IU/μl. The test runs were considered valid if all controls met the quality standards and for standard curve R2 ≥ 0.98, in accordance with the manufacturer's instructions.
For samples with positive HBsAg and anti-HCV serology, respectively HBV DNA (Cobas HBV, Roche Diagnostics, GmbH, Germany) and HCV RNA (Abbott RealTime HCV, Abbot diagnostics, USA) were quantified. HIV-1 viral load (HIV VL) tests were performed by using automated systems Abbott m2000 RealTime System version 5.00 (Abbott Molecular Inc., USA) and/or Roche cobas 4800 test version 1.2.0. (Roche Diagnostics GmbH, Germany).
CD cells quantificationThe absolute count (AC) of lymphocyte subpopulations were determined by flow cytometry using four color BD Multitest CD3/CD8/CD45/CD4 and standard TRUCount tubes (BD Biosciences, FACSCanto II). The referent minimal-average-maximal (min-aver-max) values of absolute number for different subtypes were: 1000-1800-2800 [cells/µl]; 700-1200-2500 [cells/µl]; 400-700-1600 [cells/µl]; 11-24-38 [cells/µl], respectively for CD45, CD3, CD4 and CD8.
Statistical analysisQuantitative variables were summarized by mean and standard deviation (mean±SD) or median (25th - 75th percentile), based on the sample distribution. Qualitative variables are presented as numbers and percentages (n, %). The Kolmogorov-Smirnov test was applied to evaluate if a normal distribution could be assumed. Differences between variables among groups were tested using t-test, or z-test when appropriate, for independent-samples. Jonckheere-Terpstra rank-based nonparametric test (TJT) was applied to determine a statistically significant trend between infection type and transmission routes. Logistic regression was performed to determine the variables independently associated with HEV infection and presented as odds ratio (OR) and 95% confidence intervals (CI) of the OR. Categorical variables were compared using χ2 test and one-way ANOVA (for multiple comparison). A 2-sided p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics v. 26 software (IBM Corp., Chicago, IL, USA).
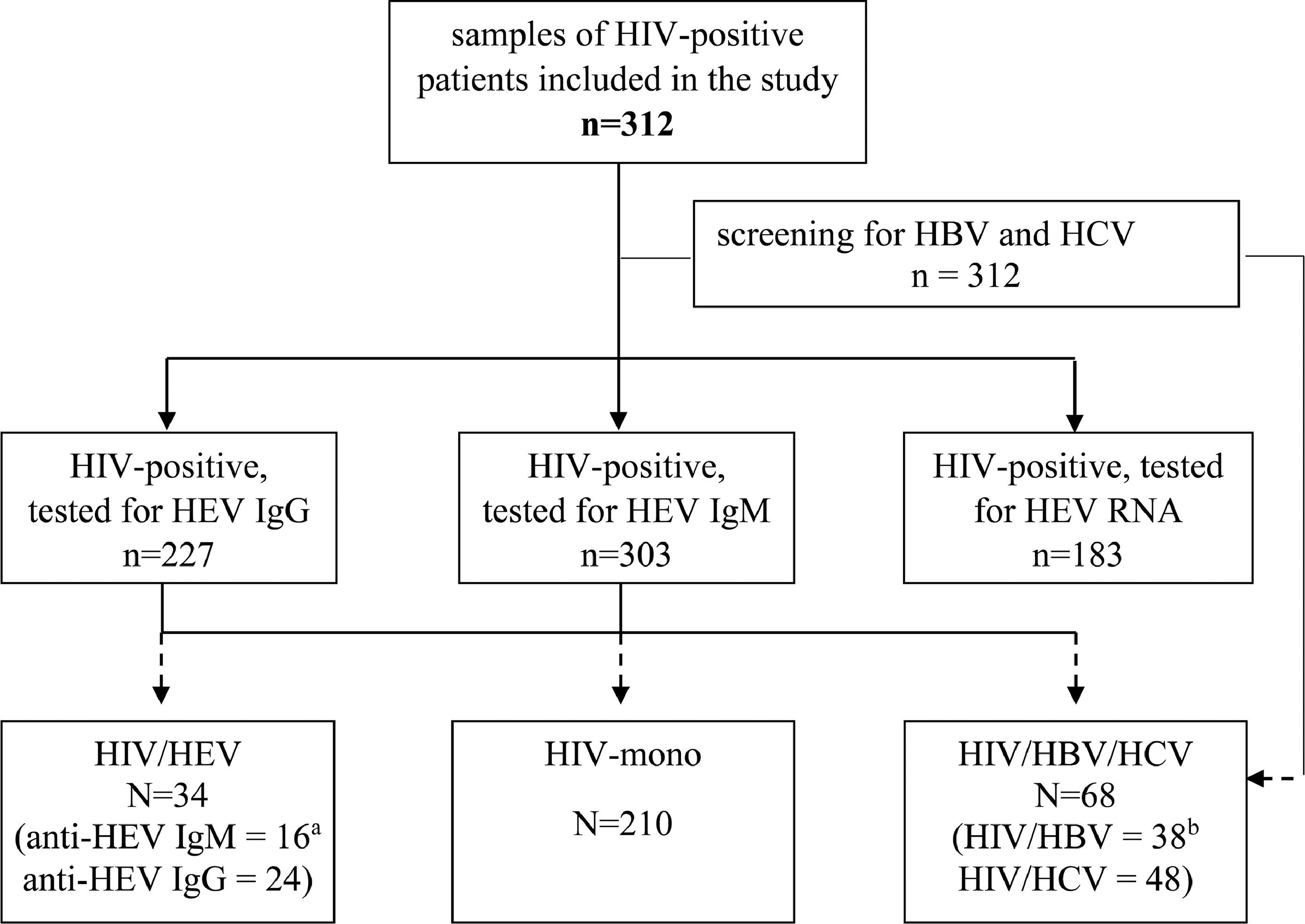
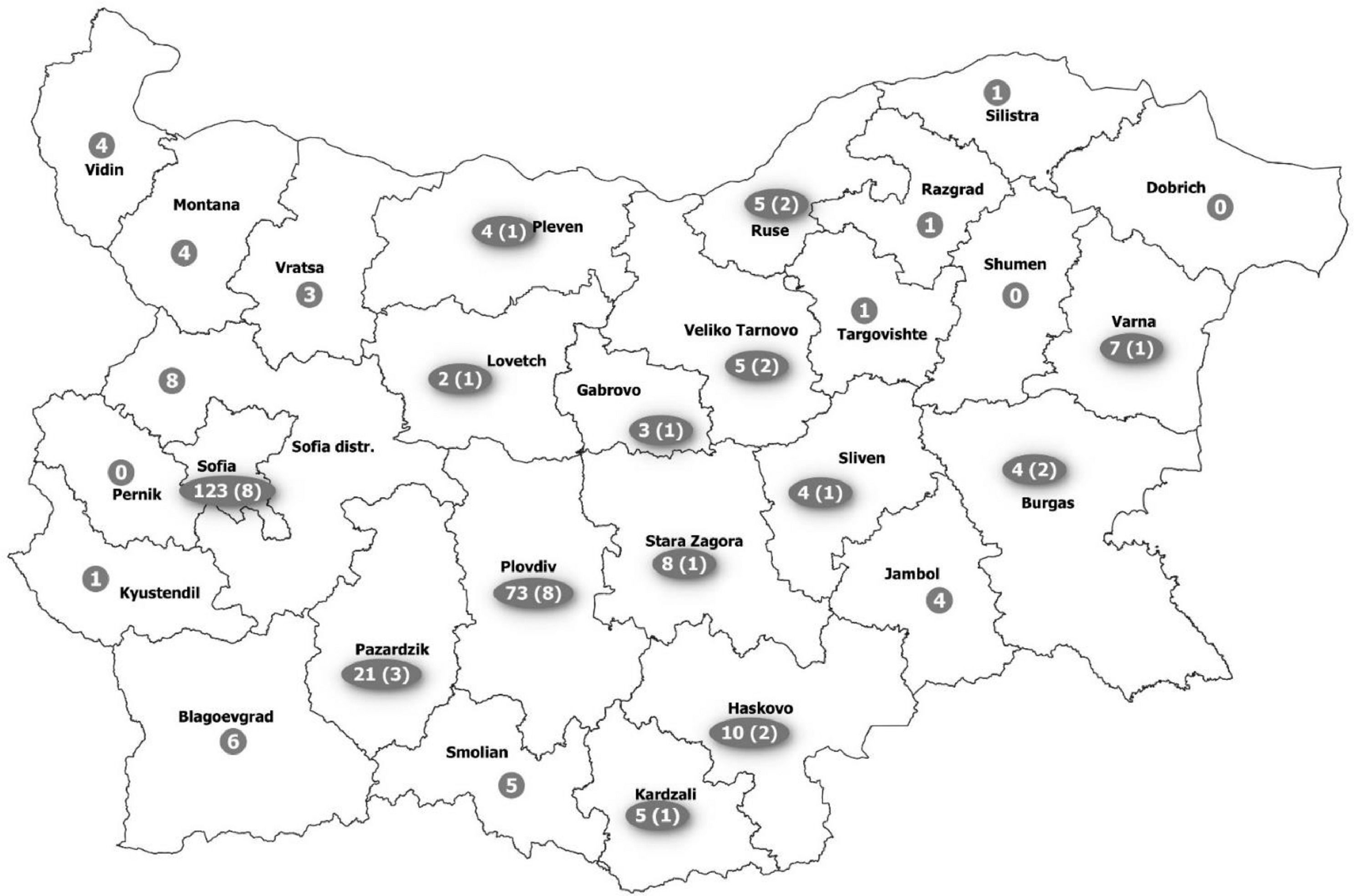
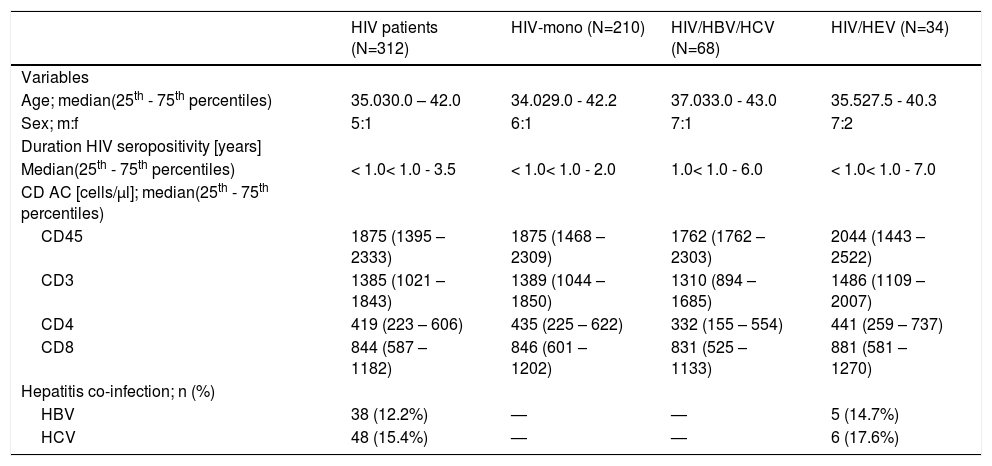
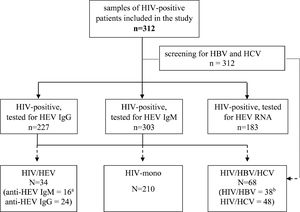
ResultsCharacteristics of the studied HIV-populationSerum samples from 312 HIV-infected patients (Fig. 1) from all over the country were retrospectively evaluated (Fig. 2). The baseline characteristics of HEV antibodies positive and negative patients are summarized in Table 1. The median age of HIV-infected pateints was 35 years (range 8 – 69) and male to female ratio of 5:1, 84.6% (264/312) men and 15.4% (48/312) women. With regards to the place of residence, 228/312 (73.1%) were living in cities over 100,000 population. The main transmission routes for HIV infection were homosexual sex in 45.2% (137/303) and heterosexual sex in 39.6% (120/303). Intravenous drug use (IDU) was recorded for 14.5% (44/303) of the HIV-infected patients, and 0.7% (2/303) was vertically infected. The median known duration of HIV seropositivity was less than one year. The longest period of HIV seropositivity was 15 years. For 51.9% (162/312) of the patients, HIV seropositivity was confirmed up one year before and for 9.0% (28/312) over 10 years. HIV viral load was below lower limit of detection (LOD <1.3 or <1.6 Log [copies/ml]) in 32.7% (102/312) of the tested samples, and greater than 3 Log [copies/ml] in 59.3% (185/312). Regarding HBV and HCV coinfections, 12.2% of the HIV-infected patients was HBV infected (HBsAg and/or HBV DNA positive) and 15.4% was HCV infected (anti-HCV and/or HCV RNA positive). The median counts of CD subtypes were: CD45 1875 (1395 – 2333), lowest value of 90; CD3 1385 (1021 – 1843), min value 77; CD4 419 (223 – 606), min value 2; and for CD8 cell count 844 (587 – 1182) (Table 1). The observed frequencies for cell count less than minimal values of different CD subtypes were: 13.8% for CD45 (<1000 cells/μl); 11.2% - CD3 (<700 cells/μl); 45.8% – CD4 (<400 cells/μl); and 2.6% for CD8 (<200 cells/μl). The frequencies of counts over maximal values were respectively: 13.8% for CD45 (> 2800 cells/μl); 8.7% - CD3 (>2500 cells/μl); 0.3% – CD4 (>1600 cells/μl); and 30.4% for CD8 (> 1100 cells/μl).
Flow diagram of the study and the differentiation of evaluated groups. HIV = human immunodeficiency virus; HBV = hepatitis B virus; HCV = hepatitis C virus; IgM = immunoglobulin M; IgG = immunoglobulin G; HIV mono = HIV mono-infected; HIV/HBV/HCV = HIV positive for HBV and/or HCV, but HEV negative; HIV/HEV = HIV positive for HEV. Legend: a The number is for anti-HEV IgM positive only or for anti-HEV IgG positive; b The number is for HIV/HBV co-infected or HIV/HCV co-infected.
Group-based descriptive characteristics of HIV-infected patients.
HIVmono = HIV mono-infected; HIV/HBV/HCV = HIV positive for HBV and/or HCV, but HEV negative; HIV/HEV = HIV positive for HEV; CD45, CD3, CD4, CD8 = lymphocyte subpopulations; AC = absolute count; HBV = hepatitis B virus; HCV = hepatitis C virus
Of the 312 serum samples from HIV-infected patients, included in the analysis, 34 (10.9%) were positive at baseline for HEV antibodies (Fig. 1). Out of this 34 HIV/HEV positive samples 24 (70.6%) were anti-HEV IgG positive and 16 (47.1%) anti-HEV IgM positive. The simultaneous anti-HEV IgM and anti-HEV IgG positive results were detected in 6 (17.6%) samples. None of the tested samples turned out positive for HEV RNA. The median age of HIV/HEV positive patients was 35 years, ranging from 16 to 62 years and the male to female ratio 7:2 was close to the retrospective HIV-infected group. The median duration of HIV seropositivity was <1 year, and the longest period of HIV seropositivity was 14 years. HBV coinfection was detected in 14.7%, and HCV in 17.6% of the HEV cohort (Table 1). Out of the HIV/HEV positive individuals, 64.7% were from cities with population over 100,000. The documented HIV transmission routes in HIV/HEV group were heterosexual (38.2%), homosexual (35.3%), IDU (23.5%), and vertical (2.9%). For 47.06% of HEV positive patients, HIV infection was diagnosed up to one year before, and for 20.59% between five to 10 years. The HIV VL was higher than 3 Log [copies/ml] in 61.76% of HIV/HEV positive patients. As far as the immunological status is concerned, the highest frequencies for different CD subtypes were in the average range (≥minTable 2).
Frequencies of evaluated variables associated with HEV seropositivity. Values expressed as number of cases (N) and percent (%).
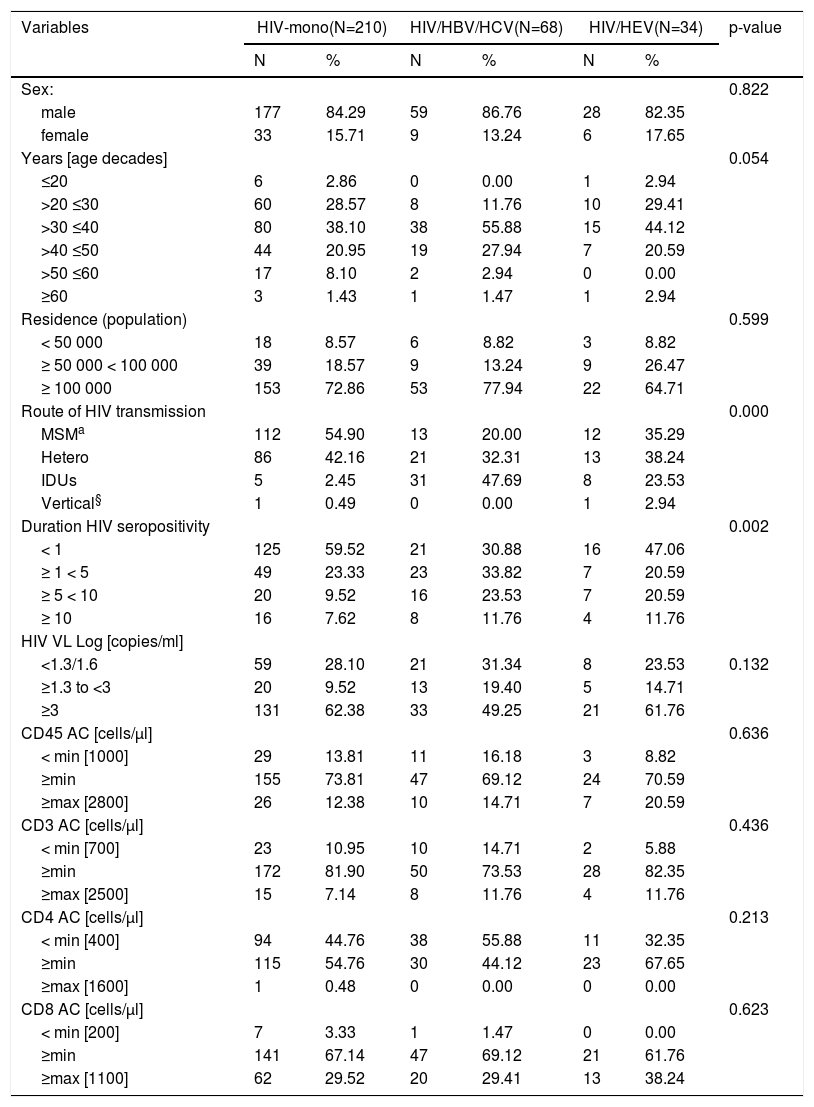
| Variables | HIV-mono(N=210) | HIV/HBV/HCV(N=68) | HIV/HEV(N=34) | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Sex: | 0.822 | ||||||||
| male | 177 | 84.29 | 59 | 86.76 | 28 | 82.35 | |||
| female | 33 | 15.71 | 9 | 13.24 | 6 | 17.65 | |||
| Years [age decades] | 0.054 | ||||||||
| ≤20 | 6 | 2.86 | 0 | 0.00 | 1 | 2.94 | |||
| >20 ≤30 | 60 | 28.57 | 8 | 11.76 | 10 | 29.41 | |||
| >30 ≤40 | 80 | 38.10 | 38 | 55.88 | 15 | 44.12 | |||
| >40 ≤50 | 44 | 20.95 | 19 | 27.94 | 7 | 20.59 | |||
| >50 ≤60 | 17 | 8.10 | 2 | 2.94 | 0 | 0.00 | |||
| ≥60 | 3 | 1.43 | 1 | 1.47 | 1 | 2.94 | |||
| Residence (population) | 0.599 | ||||||||
| < 50 000 | 18 | 8.57 | 6 | 8.82 | 3 | 8.82 | |||
| ≥ 50 000 < 100 000 | 39 | 18.57 | 9 | 13.24 | 9 | 26.47 | |||
| ≥ 100 000 | 153 | 72.86 | 53 | 77.94 | 22 | 64.71 | |||
| Route of HIV transmission | 0.000 | ||||||||
| MSMa | 112 | 54.90 | 13 | 20.00 | 12 | 35.29 | |||
| Hetero | 86 | 42.16 | 21 | 32.31 | 13 | 38.24 | |||
| IDUs | 5 | 2.45 | 31 | 47.69 | 8 | 23.53 | |||
| Vertical§ | 1 | 0.49 | 0 | 0.00 | 1 | 2.94 | |||
| Duration HIV seropositivity | 0.002 | ||||||||
| < 1 | 125 | 59.52 | 21 | 30.88 | 16 | 47.06 | |||
| ≥ 1 < 5 | 49 | 23.33 | 23 | 33.82 | 7 | 20.59 | |||
| ≥ 5 < 10 | 20 | 9.52 | 16 | 23.53 | 7 | 20.59 | |||
| ≥ 10 | 16 | 7.62 | 8 | 11.76 | 4 | 11.76 | |||
| HIV VL Log [copies/ml] | |||||||||
| <1.3/1.6 | 59 | 28.10 | 21 | 31.34 | 8 | 23.53 | 0.132 | ||
| ≥1.3 to <3 | 20 | 9.52 | 13 | 19.40 | 5 | 14.71 | |||
| ≥3 | 131 | 62.38 | 33 | 49.25 | 21 | 61.76 | |||
| CD45 AC [cells/µl] | 0.636 | ||||||||
| < min [1000] | 29 | 13.81 | 11 | 16.18 | 3 | 8.82 | |||
| ≥min | 155 | 73.81 | 47 | 69.12 | 24 | 70.59 | |||
| ≥max [2800] | 26 | 12.38 | 10 | 14.71 | 7 | 20.59 | |||
| CD3 AC [cells/µl] | 0.436 | ||||||||
| < min [700] | 23 | 10.95 | 10 | 14.71 | 2 | 5.88 | |||
| ≥min | 172 | 81.90 | 50 | 73.53 | 28 | 82.35 | |||
| ≥max [2500] | 15 | 7.14 | 8 | 11.76 | 4 | 11.76 | |||
| CD4 AC [cells/µl] | 0.213 | ||||||||
| < min [400] | 94 | 44.76 | 38 | 55.88 | 11 | 32.35 | |||
| ≥min | 115 | 54.76 | 30 | 44.12 | 23 | 67.65 | |||
| ≥max [1600] | 1 | 0.48 | 0 | 0.00 | 0 | 0.00 | |||
| CD8 AC [cells/µl] | 0.623 | ||||||||
| < min [200] | 7 | 3.33 | 1 | 1.47 | 0 | 0.00 | |||
| ≥min | 141 | 67.14 | 47 | 69.12 | 21 | 61.76 | |||
| ≥max [1100] | 62 | 29.52 | 20 | 29.41 | 13 | 38.24 | |||
HIV mono = HIV mono-infected; HIV/HBV/HCV = HIV positive for HBV and/or HCV, but HEV negative
HIV/HEV = HIV positive for HEV; Hetero = heterosexual route of HIV transmission
MSM = men who have sex with men route of transmission
IDU = injection drug use route of transmission
HIV VL = HIV viral load; CD45, CD3, CD4, CD8 = lymphocyte subpopulations; AC = absolute count
Legend: The categories equal to zero or one is not used in comparisons
To evaluate the relation of different factors (variables) among the three subgroups - HIV-mono, HIV/HVB/HCV, and HIV/HEV, a comparative analysis was performed (Table 2). Males were more affected in comparison to females in all groups, respectively, 84.29%, 86.76% and 82.35% vs. 15.71%, 13.24% and 17.65% (chi-square=0.393; p=0.822). The highest percentage of positive samples were detected in the age group > 30 to ≤ 40, respectively, 38.10%, 55.88% and 44.12% for HIV-mono, HIV/HVB/HCV, and HIV/HEV groups, with no statistical significance (p=0.054). There were no HIV/HEV samples detected in age group > 50 to ≤ 60. The predominant number of HIV-infected patients among the studied groups were from cities with over 100,000 population. Significant association was found with respect to the reported HIV transmission routes (p < 0.001). Man who have sex with man (MSM) was the predominant HIV transmission route mono-infected patients (54.90%) in comparison with 20.00% (13/64) among HIV/HBV/HCV (z=4.9; p<0.001) and 35.29% (12/34) in HIV/HEV group (z=2.1; p=0.034). The heterosexual route of transmission was almost equally distributed among the groups (42.6%, 32.31% and 38.24%, respectively). There were less injecting drug users (IDUs) among HIV-mono group 2.45% (5/204) vs 23.53% (8/34) for HIV/HEV group and 47.69% (31/64) for HIV/HBV/HCV group (z=2.3; p=0.023). Consequently, to evaluate differences in samples frequencies according to routes of HIV transmission between HIV-mono, HIV/HBV/HCV and HIV/HEV groups, statistical analysis using non-parametric Jonckheere Terpstra test (TJT) for ordered alternatives was performed. Results demonstrated statistically significant trend of HIV mixed infection with routes of transmission different from MSM - heterosexual in HIV/HEV group and IDU in HIV/HBV/HCV group (TJT=8400.50, z=4.19, p<0.0001).
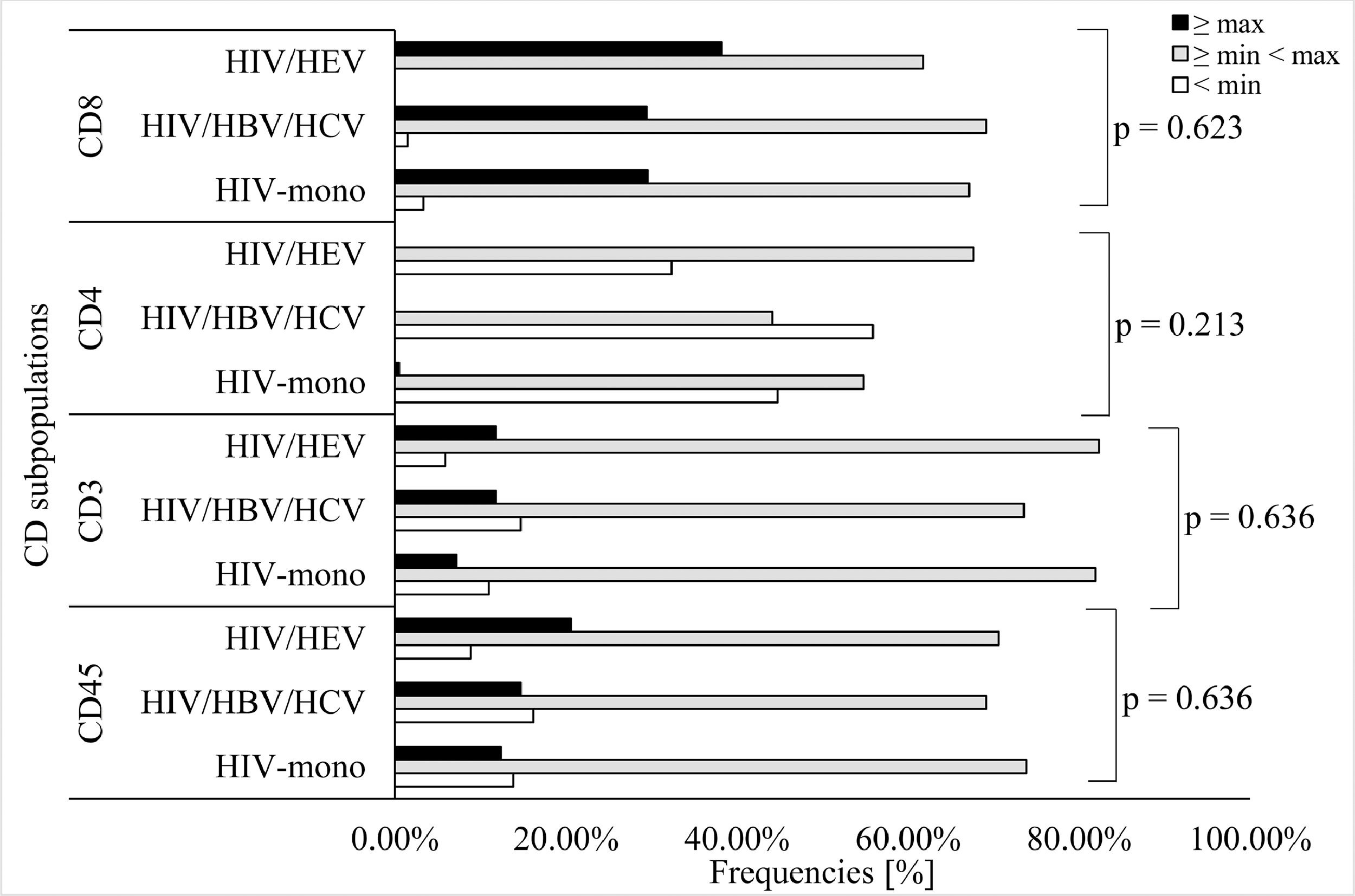
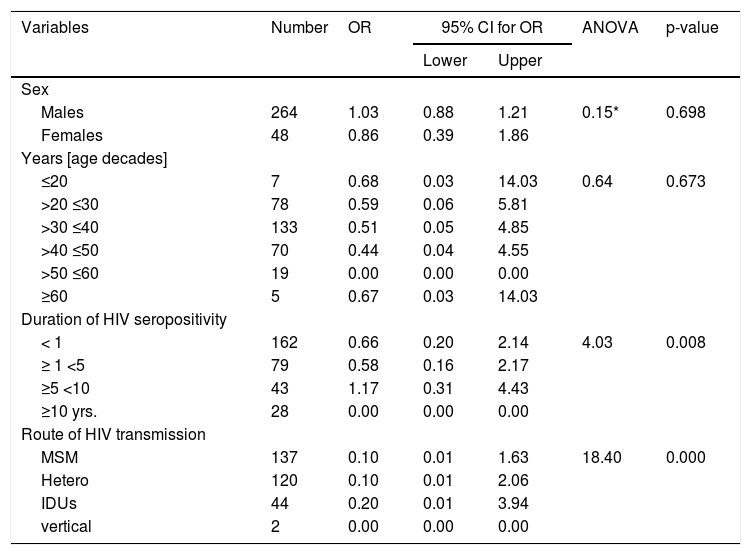
For all tested groups, most of the samples originated from patients whose HIV positivity was laboratory confirmed up to 1 year before (59.52%, 30.88%, and 47.06%) (Table 2). Significant association between duration of HIV seropositivity (for periods < 1 year and ≥ 5 < 10 years) and type of infection was observed (p = 0.002). To determine the differences in the frequency of the categorical variables a logistic regression analysis was performed. In univariate logistic regression analysis, duration of HIV diagnosis between 5 to 10 years (OR = 1.17; 95%CI = 0.31–4.43) and being IDUs (OR = 0.20; 95%CI = 0.01–3.94) were risk factors associated with HEV seropositivity (Table 3). No significant difference in OR was observed for HEV seropositivity and sex and age groups. The risk factors duration of HIV diagnosis and transmission route were not independently associated with HEV infection in a multivariate logistic regression analysis. CD45, CD3, CD4, and CD8 cells counts were not significantly different among the three groups (Table 2, Fig. 3). Overall, in all groups most of the samples had CD45 and CD3 cells counts in average range, respectively 73.81%, 69.12%, and 70.59% for CD45, and 81.90%, 73.53%, and 82.35% for CD3. For CD4 cells count the samples were distributed between ≤ min and average (≥min
Univariate logistic regression analysis to test the association between HEV seropositivity and the associated risk factors.
| Variables | Number | OR | 95% CI for OR | ANOVA | p-value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Sex | ||||||
| Males | 264 | 1.03 | 0.88 | 1.21 | 0.15* | 0.698 |
| Females | 48 | 0.86 | 0.39 | 1.86 | ||
| Years [age decades] | ||||||
| ≤20 | 7 | 0.68 | 0.03 | 14.03 | 0.64 | 0.673 |
| >20 ≤30 | 78 | 0.59 | 0.06 | 5.81 | ||
| >30 ≤40 | 133 | 0.51 | 0.05 | 4.85 | ||
| >40 ≤50 | 70 | 0.44 | 0.04 | 4.55 | ||
| >50 ≤60 | 19 | 0.00 | 0.00 | 0.00 | ||
| ≥60 | 5 | 0.67 | 0.03 | 14.03 | ||
| Duration of HIV seropositivity | ||||||
| < 1 | 162 | 0.66 | 0.20 | 2.14 | 4.03 | 0.008 |
| ≥ 1 <5 | 79 | 0.58 | 0.16 | 2.17 | ||
| ≥5 <10 | 43 | 1.17 | 0.31 | 4.43 | ||
| ≥10 yrs. | 28 | 0.00 | 0.00 | 0.00 | ||
| Route of HIV transmission | ||||||
| MSM | 137 | 0.10 | 0.01 | 1.63 | 18.40 | 0.000 |
| Hetero | 120 | 0.10 | 0.01 | 2.06 | ||
| IDUs | 44 | 0.20 | 0.01 | 3.94 | ||
| vertical | 2 | 0.00 | 0.00 | 0.00 | ||
Legend: p-value of <0.05 was considered statistically significant
In this retrospective cohort study of HIV-infected patients from Bulgaria, the documented HEV seropositivity was 10.9%. An association between HEV seroprevalence and HIV route of transmission was observeded, within the compared groups and a significant increase of seroprevalence was detected in those whose route of transmission heterosexual sex. HEV seroprevalence increased with age for the young (from 20 to 30 years) and middle age groups (from 30 to 40 years), but associations were non-significant. Differences in HEV seroprevalence within the compared groups for other demographic and viral factors – sex, settlement, duration of HIV-seropositivity, presence of HBV or HCV co-infection, were not detected. The immunological status, represented by CD subtypes cell count, was not a factor for increased seroprevalence. Additionally, no active or chronic HEV infection was found, as all tested for HEV RNA samples were negative.
The global anti-HEV IgG seroprevalence in the general population is 12.47% and 9.31% in Europe.18 Among HIV-infected individuals, HEV seroprevalence varies between 40% for Africa and Asia, and 10% for European countries.7 In Southern Bulgaria, the HEV prevalence varies from 9.04% for outpatients13 to 25.9% for blood donors.14 In the present study in a cohort of 312 HIV-infected patients the observed prevalence was 10.9%, which is lower compared with blood donors and hemodialysis patients (14.7%). An anti-HEV IgG seropositivity of 2.6% was reported in Swiss HIV patients,19 7.3% in Greece,20 and 38.7% in France.21 HIV-infected population in Bulgaria is not at high risk for infection with HEV. The increasing HEV seropositivity in patients most recently HIV diagnosed (<1 year) may be explained by a higher number of tests performed in HIV-infected samples within this period.
Among the HIV/HEV-positive group, the male to female ratio was 7:2, which correlated to the sex distribution in control groups. The higher representativeness of male sex can be explained by the fact that HIV infection affects more men, and men had higher rates than women in all age groups, except in persons under 15 years.22 At the same time, male sex in middle age and elderly groups is a factor associated with increased HEV seroprevalence among general population.23 This explains the much higher number of HEV positive men compared to women. The tendency of increasing number of positive samples with age was documented for all compared cohorts, and the highest rates were detected in the middle age group (>30 ≤ 40), which could be due to increasing probability of this age group to be exposed to different infectious agents.24 In most studies, the HEV prevalence is age dependent, with the highest percentage among people over 50 years.25 Such tendency of increasing prevalence with age was documented and for Bulgarian population by Theoharov et.al.,13 where the authors detected an irregularity - decrease of the anti-HEV IgG prevalence, in the age group from 50 to 59 years. In the present study, HEV seropositivity decreased in the age groups above 40 years with no positive samples detected in the age group >50 to ≤60. An inversed association between HEV prevalence among HIV-infected patients born before 1970 was observed by Alberts et al.26. This decrease in HEV seroprevalence for the Bulgarian population, including HIV-infected persons, could be explained by the life time dependence of the risk for HEV infection,27 but it needs further in-depth studies. Most of the HIV-infected patients in our study were living in cities with population over 100,000. This finding is in line with the conclusions of O'Laughlin et al.28 that barriers to care included distance, cost, unemployment, and the stigma associated with HIV infection, which are easier to overcome in the large regional cities, where the centers for treatment and follow-up of HIV patients are based.
Different routes of transmission for human HEV strains have been established: waterborne, foodborne, blood borne, vertical, person-to-person (uncommon), nosocomial (a single outbreak reported to date), and via liver transplantation.29 HIV-1 is transmitted by sexual contact across mucosal surfaces, by maternal-infant exposure, and by percutaneous inoculation.30 In the present study, HIV transmission routes were significantly different within the evaluated groups – HIV/HEV and HIV/HBV/HCV vs HIV-mono (TJT=8400.50, z=4.19, p<0.0001). For the HIV/HEV group, all four types of HIV transmission routes were documented and the predominant modes were heterosexual (38.2%), followed by MSM (35.5%), and IDU (23.5%). While for HIV/HBV/HCV the predominant route of HIV transmission was IDU (47.7%) and for HIV-mono was MSM (54.9%). According to data reported in 2019 by ECDC, in the EU/EEA MSM were the predominan transmission category (39%) of all new HIV diagnoses, followed by 33% of heterosexual route of transmission, and only 4% for IDU.17 HIV transmission due to injection drug use was responsible for 37.4% of all newly diagnosed HIV cases in Bulgaria and in 2016 IDU and MSM contributed with 88% of new diagnoses.31 This is consistent with the predominant route of homosexual transmission in a group of HIV monoinfected patients in this study. The significant association of HEV IgG seropositivity and HIV infection in IDU, but its absence among MSM, was detected and by Alberts et al.26. According to 2019–2018 data of the ECDC for the WHO European region, 4% of all newly diagnosed HIV and 5% of those with known route of HIV transmission were attributed to injecting drug use. In Bulgaria, IDU is the third transmission route after MSM and heterosexual sex.22 At the same time, the number of reports about transfusion-transmitted HEV is increasing in the last years.32 Thus, despite the absence of a statistically significant difference, IDU with needle sharing and the burden of intravenous drug use among the HIV-infected Bulgarian population is a factor that may be associated with HEV infection among the HIV patients. One could speculate that the factor associated with the spread of HEV is the ability of the virus to be transmitted through different routes of transmission.
Finally, there was no significant association between HIV viral load, immunological status (CD45, CD3, CD4 and CD8 actual cells count) and HEV seropositivity in the HIV-infected patients. In all groups CD45 and CD3 cells counts varied in average range (from ≥min to33 At the same time, HEV infection does not change the proportion of CD4+ and CD8+ cells in peripheral blood mononuclear cells34 and it can be assumed that CD8+ are essential for HEV clearing.35 This could explain why none of the tested samples in this study were positive for HEV RNA.
A strength of this study are the cohort-based HEV negative control groups. The main limitation is that this was a retrospective cohort study with a limited number of patients in some of the groups, which could result in a lack of uniformity and statistical power. Unfortunately, there was no information on biochemical characteristics of the HIV-infected patients, which did not allow in-depth analysis of factors associated with HEV prevalence.
In conclusion, for the first time the HEV seroprevalence among the Bulgarian HIV-infected population was analyzed. The main factor associated with HEV seropositivity among HIV-infected patients was the ability of the HEV to be transmitted through different routes in contexts of patients’ behavior.



![Prevalence [%] of different CD subpopulations within HIV-mono, HIV/HBV/HCV and HIV/HEV cohortsLegend: P-values were calculated by chi-square test or Fisher Prevalence [%] of different CD subpopulations within HIV-mono, HIV/HBV/HCV and HIV/HEV cohortsLegend: P-values were calculated by chi-square test or Fisher](https://static.elsevier.es/multimedia/14138670/0000002600000001/v1_202203110818/S1413867022000186/v1_202203110818/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95uaF0+42b+pWE4hY44gaZY=)


