A natural and biocompatible fibrin microsphere is one of the most promising dual delivery vehicle as compared to other traditionally designed delivery modalities. It represents sustained delivery of encapsulated drug and is easily biodegradable in the blood circulation. In the present study, we evaluated the systemic augmentation of the antifungal activity of amphotericin B loaded in fibrin microsphere (AMB-fibrin microsphere) against cryptococcosis in Swiss albino mice. Mice infected with Cryptococcus neoformans were treated with 0.5mg/kg AMB-fibrin microsphere that was given alternately for 7 days. The antifungal potential of AMB-fibrin microsphere was assessed on the basis of reduction of cfu count in the systemic circulation and various vital organs of infected mice. The formulation was found to be highly effective in reducing intracellular pathogen from the experimental animals where fibrin microsphere significantly controlled the release of amphotericin B for longer time duration. The AMB-fibrin microsphere chemotherapy was significantly more effective than free amphotericin B in reducing the fungal burden and showed better survival efficacy (p<0.05). The current study demonstrating the use of novel amphotericin B loaded fibrin microsphere not only imparts protection to the encapsulated amphotericin B but also offers an effective strategy to decrease the drug associated toxicities.
Cryptococcus neoformans, a medically important opportunistic fungal pathogen showing multi-organ involvement, has been isolated from various natural sources like soil and rotten vegetables.1 In human beings, C. neoformans is capable of causing pneumonia, meningitis and disseminated cryptococcosis in the presence of impaired cell mediated immunity.2,3 Experimental studies have shown partial protection and delayed-type hypersensitivity in mice after vaccination with various protein preparations.4–6 Prolonged survival and reduced fungal burden responses have also been observed after passive administration of anti-cryptococcal mAbs.7 In recent years, Cryptococcus infection has shown tremendous increase in its frequency of occurrence across the globe.8 Despite the emerging new trend of this pathogen no vaccine or immunotherapy has been found to avail complete protection against recurrence of C. neoformans challenge. Hence, the development of new chemotherapeutic strategies will be helpful in conjunction with traditional drug delivery system to combat intracellular pathogens.
Amphotericin B (AMB) is the most effective antifungal antibiotic and is recommended as treatment of choice for disseminated cryptococcal infections.8–10 A major factor limiting the use of drug is poor accessibility to the intracellular pathogens. AMB is not used usually because of high toxicity and various side effects at high doses.9,11,12 The in-house pre-designed delivery system is a strategy to combat C. neoformans with improved delivery of the drug to the target site. Various traditional drug delivery vehicles have the capability of intracellular delivery of the antifungal or antibacterial agents and control its release over a prolonged period.4,13 Several workers have shown the encapsulation of AMB into the multilamillar or unilamellar liposomes that release the drug thereby steadily reducing its toxicity.14–16
In the recent past, plasma beads have been used for delivery of various biological components. The plasma beads are composed of recipient own blood plasma and are highly biocompatible and biodegradable material.17 They can entrap both low and high molecular weight drugs and have an ability to stay longer in blood circulation. In fact, plasma beads could have an extra edge over other methods thus becoming a potential and safe drug-delivery vehicle. On the other hand, poly-lactide co-glycolide (PLGA) microsphere based system has been designed to use constant delivery of various biological components, including drugs, antibiotics and proteins, etc.18,19 The in vitro designed PLGA microspheres have revealed excellent biocompatibility. Concomitantly, due to non-toxicity, biodegradation, biocompatibility, and physiochemical properties, PLGA microspheres have a broad scope in being a potent chemotherapeutic delivery system.20
Materials and methodsChemicalsAmphotericin B, poly-lactide co-glycolide (PLGA) and polyvinyl alcohol (PVA) were purchased from Sigma–Aldrich, USA. All other chemicals used in this study were of highest purity.
Experimental animalsPathogen free Swiss albino female mice with an average weight of 18–22g were used throughout the study. The animals were fed rodent feed and filtered water ad libitum. The animals were housed in propylene cages on wood powder bedding under standard atmospheric conditions (22±1°C temperature; 12h light/12 dark photoperiod and 50–60% humidity). All experiments were performed according to the Guide for Experimental Animal Care Review Board, College of Pharmacy, King Saud University.
Experimental strain Cryptococcus neoformansC. neoformans (ATCC 24067) strain was procured from American Type Culture Collection, USA. The strain was recovered from glycerol stocks and maintained in yeast extract peptone dextrose (YPD) media while shaking at 37°C for 48h. The cell suspension was centrifuged at 5000×g for 15min at 4°C and washed thrice with sterile normal saline. After quantifying viable cell count the mice were infected intravenously with C. neoformans (1×106cfu/mouse).
Preparation of AMB suspensionAMB was diluted firstly in 5% glucose and subsequently in phosphate buffered saline (PBS) and was given intraperitoneally (ip).
Preparation of plasma beadsFor isolation of plasma, blood was collected through retro-orbital puncture from healthy mice with a heparinized capillary. Blood in heparin was centrifuged at 400×g at 4°C and plasma (supernatant) was isolated. An aliquot of plasma (250μL) was immediately used for preparation of plasma beads whereas rest of the plasma was stored at −20°C for further use. Plasma beads were prepared according to the published protocol.21
Preparation of AMB encapsulated PLGA microspherePreparation of PLGA was based on water in-oil-in-water (W/O/W) system. Microspheres were prepared by solvent evaporation technique according to the published protocol.22 For the preparation of AMB encapsulated PLGA emulsion: 5mg of AMB in 5% glucose was mixed with 190mg PLGA dissolved in dichloromethane, 10% PVA was added to primary emulsion formed by sonication. After homogenization the resulting O/W emulsion was stirred for 18h after which the formulation of AMB-PLGA microsphere was centrifuged and washed. The final purified formulation was kept in dessication after lyophilyzation at 4°C.
Preparation of fibrin microsphereThe fibrin microsphere based dual delivery system was prepared by encapsulating AMB in PLGA microsphere and further entrapped in fibrin beads according to the published protocol.4 Plasma (250μL) was mixed with the suspension of AMB encapsulated PLGA microsphere in PBS. To the mixture, calcium chloride (40mM) was also added. Aliquots (3μL) of the mixture were transferred as droplets over a parafilm covered glass slide which were then incubated at 37°C for 40min. The beads after collecting from the parafilm were washed with normal saline and then with PBS.
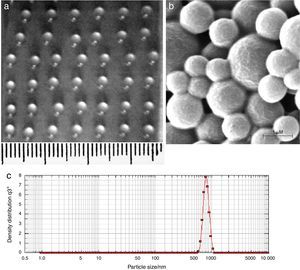
Characterization of AMB-fibrin microsphereThe particle size and surface morphologyScanning electron microscopy (SEM; Zeiss EVO 40; Carl Zeiss SMTAG, Oberkochen, Germany) of AMB-PLGA microsphere was done to examine its surface morphology. A drop of lyophilized preparation (20mM PBS, pH 7.4) was mounted on a clear glass stub, air dried and coated with gold palladium alloy. The particle size of fibrin beads bearing AMB encapsulated PLGA microspheres was determined in the dry state. The mean surface diameter was calculated by the millimeter scale.
Determination of zeta potentialThe lyophilized AMB-fibrin microsphere was reconstituted in phosphate buffer, pH 7.4 and added to the electrophoresis cell of Zetasizer nano ZS instrument (Malvern Instrument Limited, UK). The electrophoretic mobility and zeta potential values were obtained using DTS software based on M3-PALS technology. The experiments were repeated three times and the average zeta potential was calculated by taking the mean of three values.
Determination of entrapment efficiencyThe entrapment efficiency is the amount of drug entrapped in the delivery vehicle with respect to the total amount of drug used in the preparation of formulation. Published method of Moynihan et al.23 was used to determine the entrapment efficiency of AMB-PLGA microspheres and AMB-fibrin microspheres. A 10-mg sample was mixed with 1mL of 0.1N sodium hydroxide solution and shaken at 37°C to obtain a clear solution. The supernatant of the solution was centrifuged at 3000rpm for 4min and then analyzed by NanoDrop 2000 (Thermo Scientific, USA). The AMB content in PLGA microspheres and fibrin microsphere was read at 405nm.
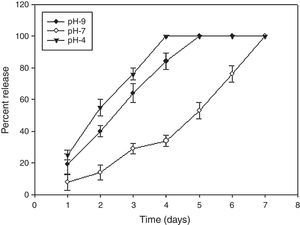
Determination of in vitro release profileThe release kinetic assay indicates stability of AMB-fibrin microsphere under different pH conditions. The release of AMB was determined at three different pH mediums viz., pH 4, pH 7, and pH 9. Measured aliquots of AMB-fibrin microsphere were taken in multiple vials to assess the release of AMB from fibrin microsphere. One mL of 20mM sterile PBS was added to each vial and incubated at 25°C. Release runs were continued for one week. At every alternate day, 100μL aliquot was taken from each vial. The aliquots were centrifuged at 9168×g for 10min and supernatants were spectroscopically read at 405nm. The calculated amount of the released AMB in different pH environment was plotted against time.
Determination of treatment dose of AMBHealthy Swiss albino mice were divided into six groups of five mice each. One group served as control and received 0.25mL PBS, ip. The remaining groups were injected with different amount of AMB. After 24h, the mortality rate of each group was determined. The 0.5mg/kg b.w. dose of free drug resulted in almost 50% survival of the treated mice. Therefore, this dose was used in present experiment. The selected dose is similar to the already reported dose.18
Antifungal therapy using AMB-fibrin microsphereAntifungal treatment was begun one day post-infection for a period of seven days. Treatment was given once in the 1st, 3rd, and 5th day post-infection. The pathogen infected mice were divided into five groups comprising of 10 animals each. The mice of group I were used as untreated control and received no treatment. Group II mice were injected with free AMB at a single dose of 0.5mg/kg b.w. ip. Group III mice were injected ip with 500μL of AMB-plasma beads. In Group IV, the mice were treated with AMB-PLGA microsphere at a dose of 0.5mg/kg b.w. in 500μL; ip. Group V mice were injected ip with 500μL of AMB-fibrin microsphere.
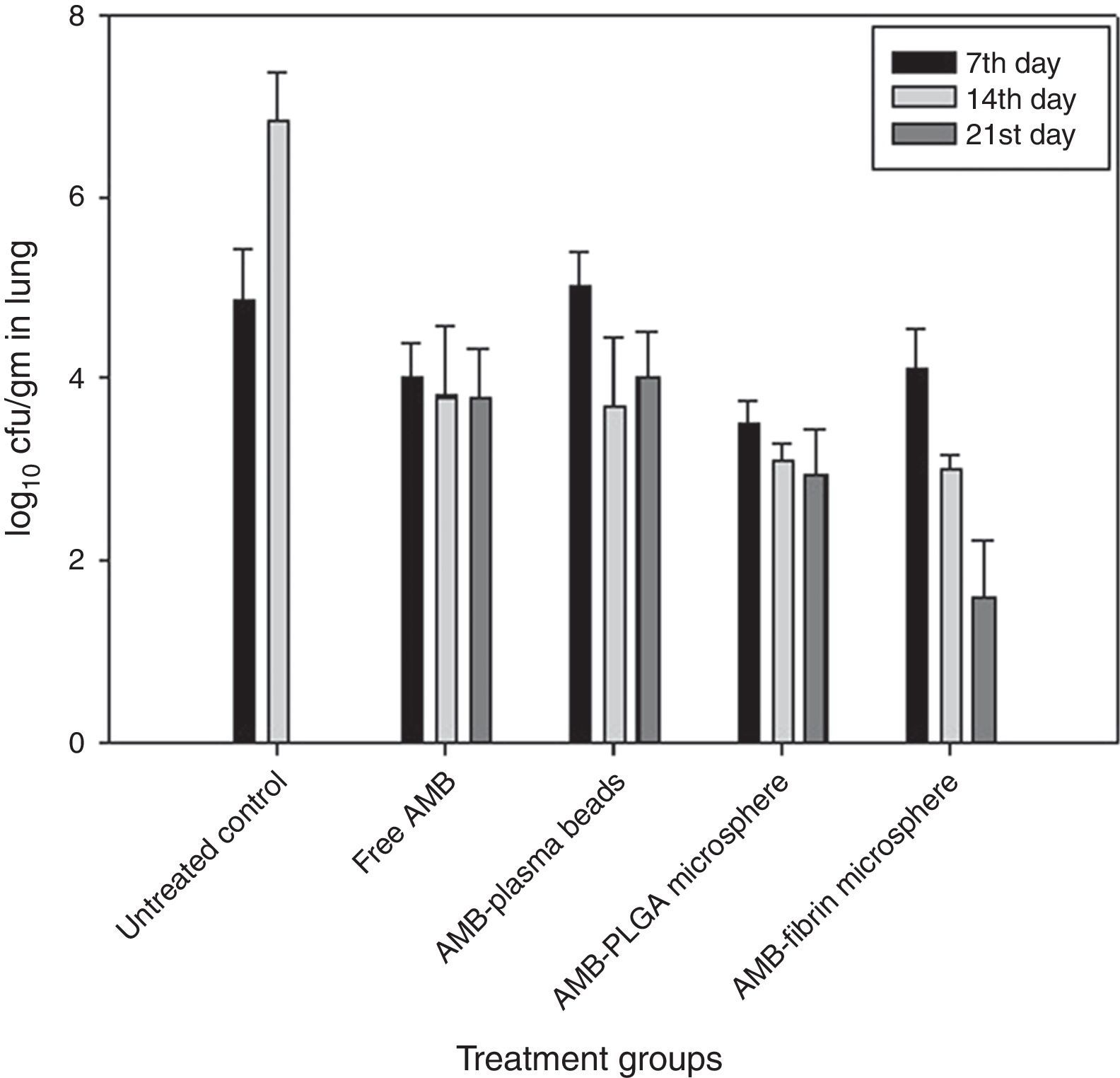
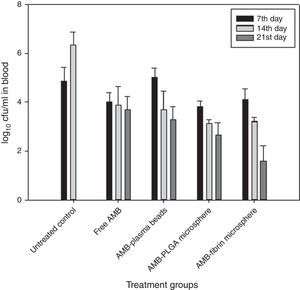
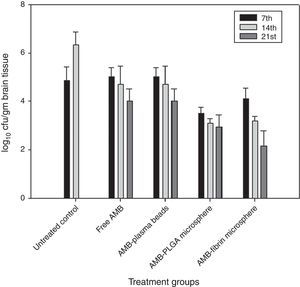
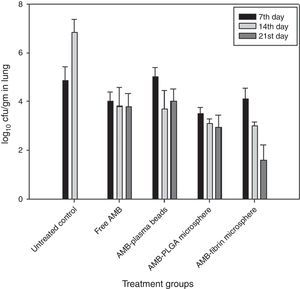
Determination of fungal burden in various vital organsThe efficacy of AMB-fibrin microsphere against severity of C. neoformans was confirmed by assessing the cfu count in various vital organs, namely lung, brain and blood. The mice from experimental groups were euthanized in the 7th, 14th and 21st day post-infection and organs were taken out aseptically. The organs were homogenized separately in 5mL PBS and an aliquot of the homogenate was plated on YPD agar plates after appropriate dilutions. The number of colonies in each group were noted after 48h of incubation at 37°C. The fungal load in each organ was calculated by multiplying with the respective dilution factor.
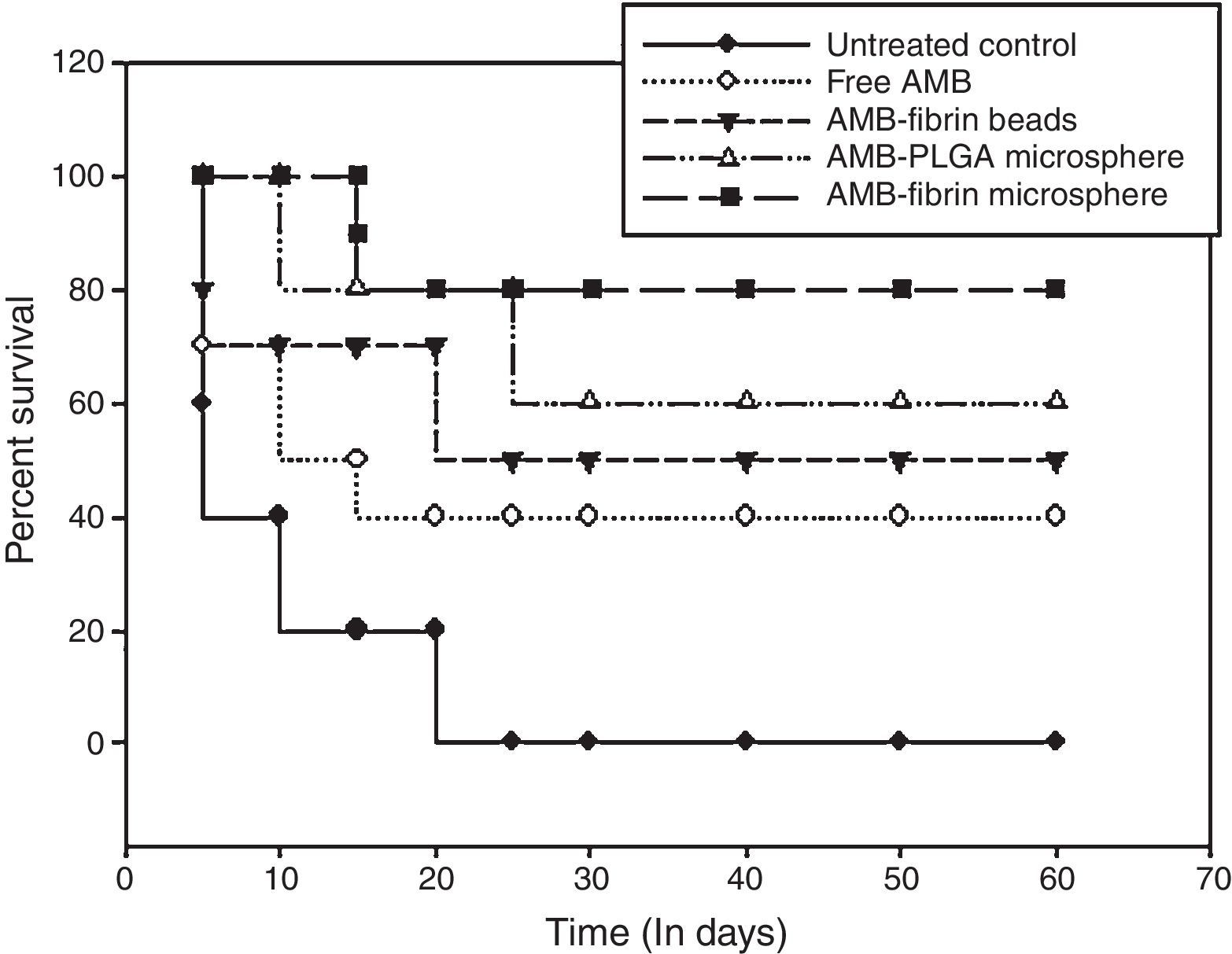
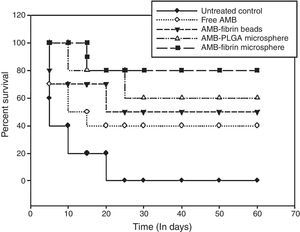
Survival studiesThe efficacy of the formulation was further confirmed by monitoring the survival of remaining mice from experimental groups. For survival studies, mortality of the mice was observed twice each day during 60 days of observation.
Statistical analysisThe data were analyzed by one-way analysis of variance (ANOVA) following Student's t-test. p values of <0.05 were considered statistically significant. All analyses were performed with SPSS v20.0 software.
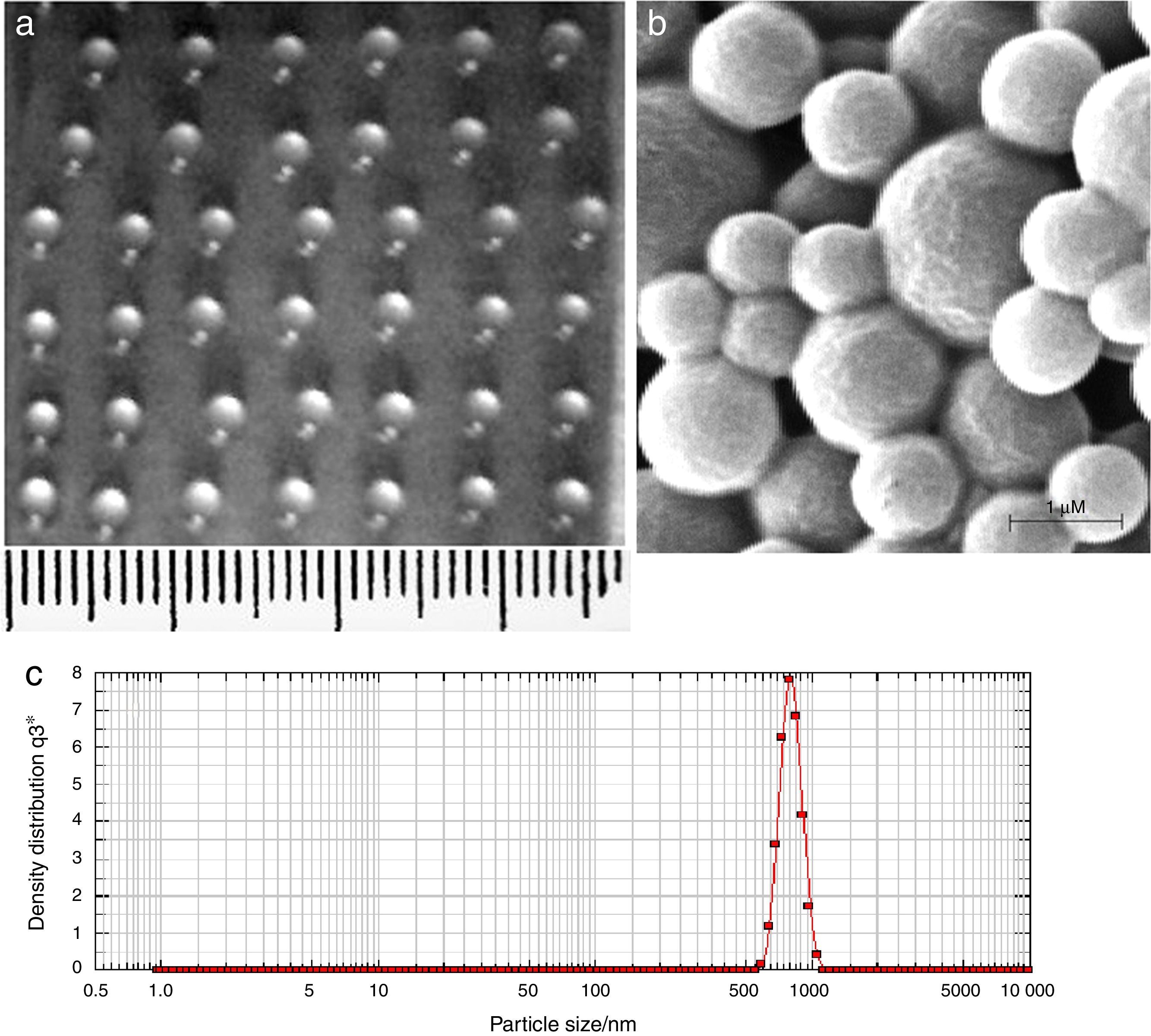
ResultsCharacterization of AMB-fibrin microsphereFig. 1a shows the fibrin microsphere beads formed with 3μL calcium-enriched plasma. The beads were spherical in shape with diameter between 1.0 and 1.5mm. Fig. 1b shows the size and surface morphology of AMB-PLGA microspheres as revealed by SEM. PLGA microspheres were spherical in structure and their size varied in the range of 300–800nm (Fig. 1b). The size of the AMB-PLGA microsphere was further confirmed with the particle size analyser and it was in good agreement with their diameter (Fig. 1c).
Entrapment efficiency, release kinetics and zeta potential of AMB-fibrin microsphereThe entrapment efficiency of AMB-PLGA microsphere and AMB-fibrin microsphere was obtained to be 65.6±2.5 and 78.31±4.7, respectively. The zeta-potential stability of AMB-fibrin microsphere was found to be −35±2.0mV.
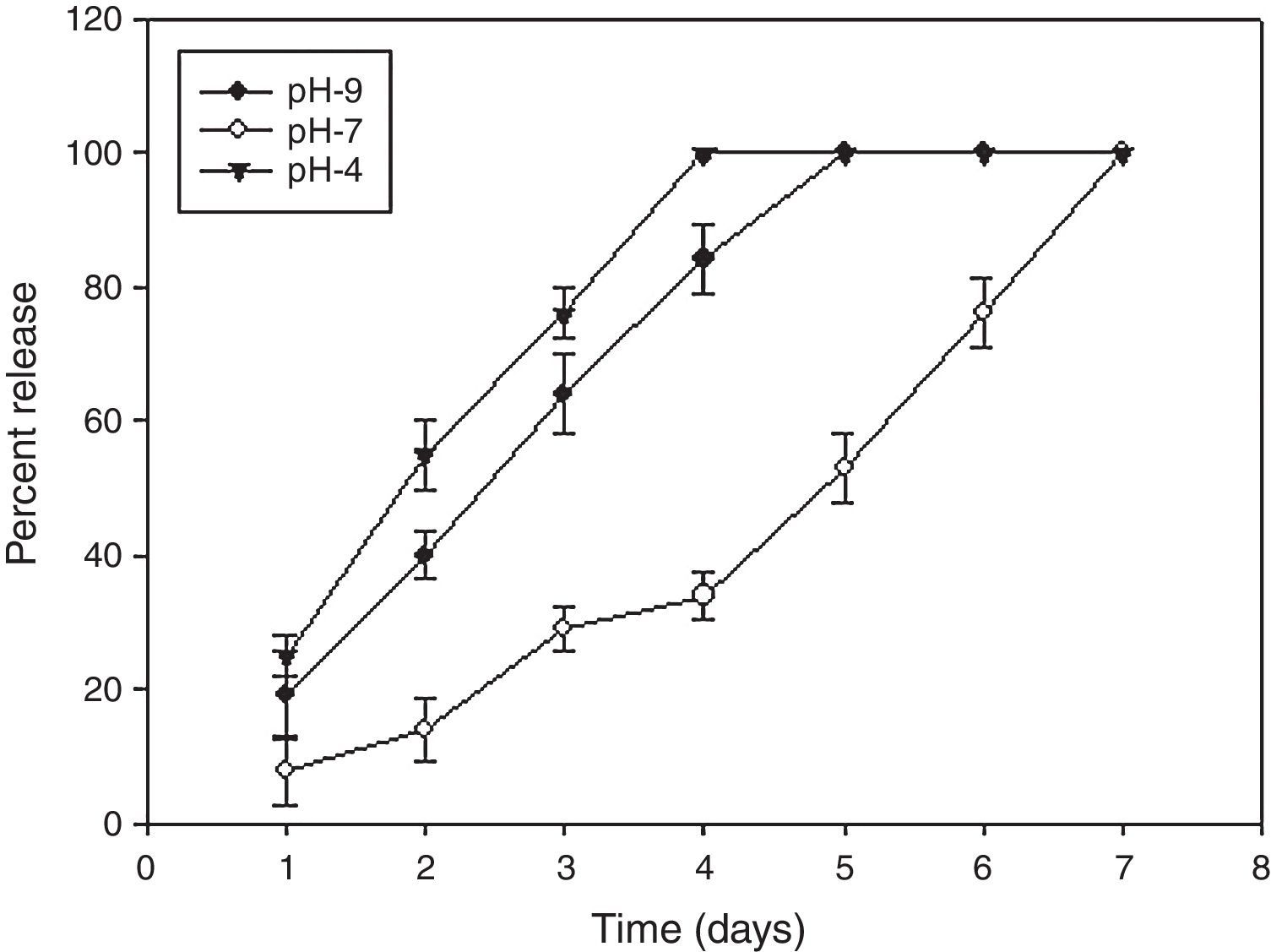
The AMB-fibrin microsphere was found to withstand both normal and alkaline conditions for a period of 48h (Fig. 2). For first two days, the formulation at pH 7 showed sustained release pattern, where only ∼10 to 30% of AMB was released from the total amount loaded in the fibrin microsphere. The rest of the 89% of AMB was released from formulation in one week. However, the whole AMB leaked out in the 4th and 5th days at pH 4 and 9, respectively.
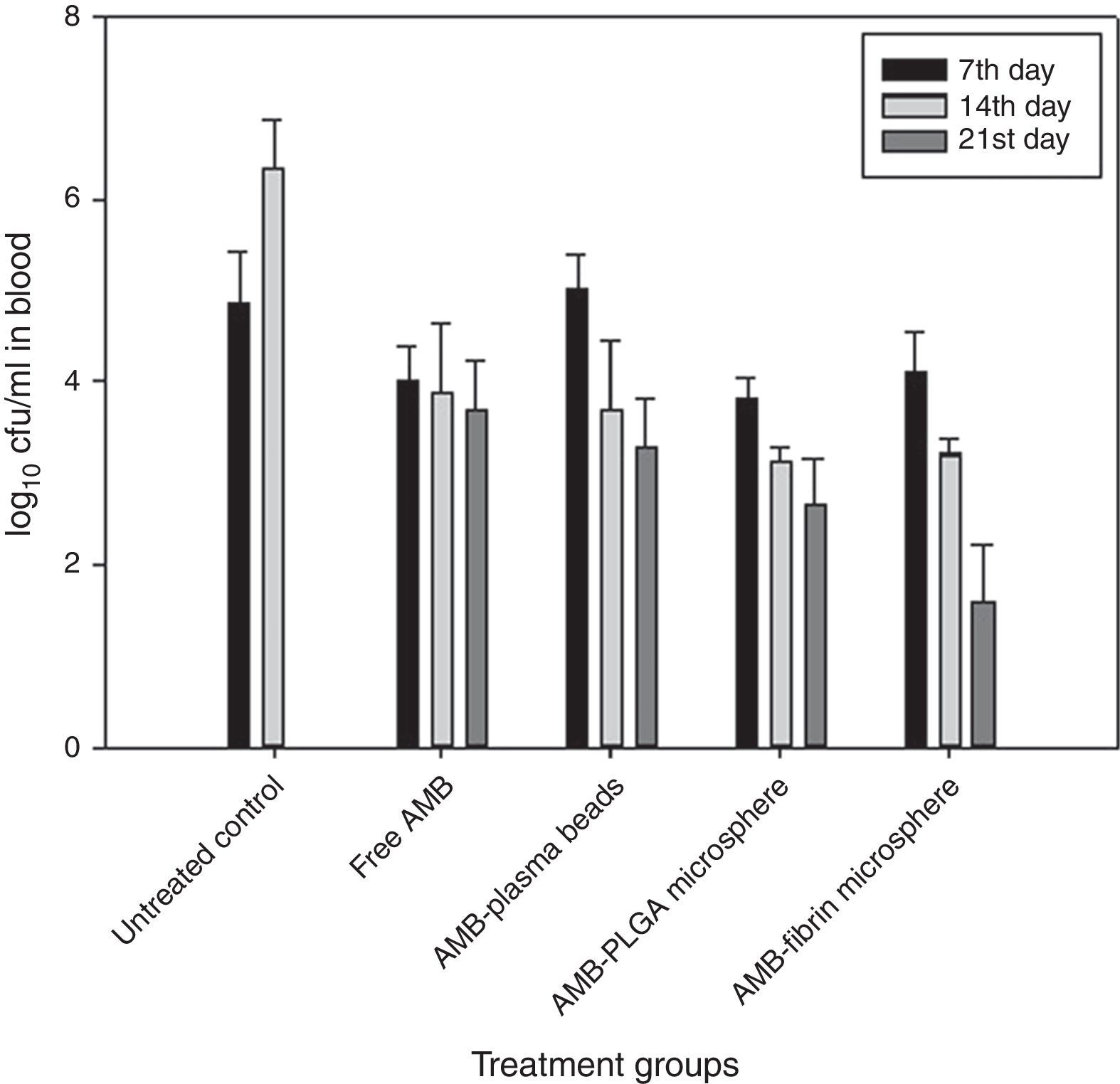
Therapeutic potential of AMB-fibrin microsphere against cryptococcosisThe effects of AMB-fibrin microsphere were investigated against C. neoformans in murine model. In the first set of experiments, the fungal burden in tissues was taken into consideration. All the mice of treatment groups were infected with 1×106cfu/mouse of C. neoformans. The antifungal therapy was started one day post-infection on alternate days. The mice were euthanized in the 7th day of therapy for determining early tissue burden and in the 14th and 21st days for determining late tissue burden. The effect of therapy on reduction in fungal burden in blood, brain, and lung is shown in Figs. 3–5, respectively. In the blood, AMB-fibrin microsphere therapy was more effective than free AMB (Fig. 3). The AMB-fibrin microsphere therapy showed significant (p<0.05) reduction in the fungal load in blood from 4.12±0.019 mean cfu on day 7 to 1.68±0.018 mean cfu on day 21 as compared to the fungal burden in untreated control (4.91±0.001 mean cfu on day 7). Free AMB was able to reduce fungal burden till day 7 only (4.00±0.026 mean cfu). In contrast, AMB-PLGA microsphere was also able to reduce the total load of C. neoformans present in blood from 3.18±0.019 mean cfu on day 7 to 2.68±0.018 mean cfu on day 21.
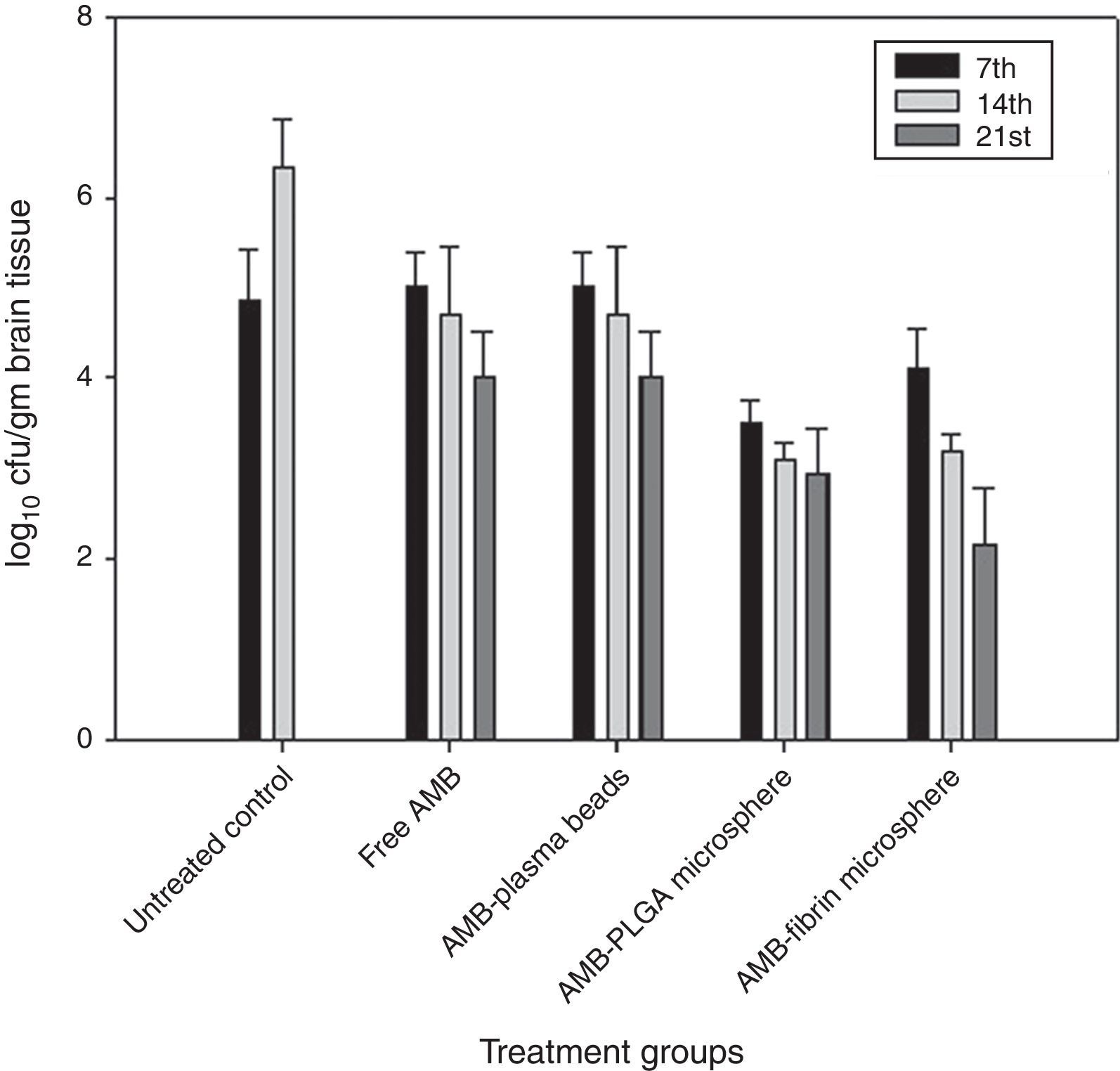
AMB-fibrin microsphere was superior at reducing fungal burden in brain (Fig. 4). The mean cfu values 4.01±0.021 (day 7), 3.23±0.021 (day 14), and 2.19±0.023 (day 21) demonstrates a significant (p<0.05) reduction in fungal burden in brain upon treatment with the formulation as compared to mean cfu value of 4.91±0.005 (day 7) and 6.34±0.003 (day 14) of untreated controls. Free AMB was unable to invoke significant antifungal response.
In the lung, the AMB-fibrin microsphere therapy was also found more effective than free AMB (Fig. 5). For C. neoformans, the fibrin microsphere as well as AMB-PLGA microsphere with the exception of free AMB and AMB-fibrin beads were significantly effective at reducing fungal burden relative to untreated controls (p<0.05). The AMB-fibrin microsphere therapy was significantly more effective (mean cfu 4.10±0.003, day7; 3.01±0.014, day 14; 1.65±0.015, day 21) than untreated controls (mean cfu 4.89±0.005, day 7; 6.87±0.007, day 14) at reducing fungal burden in the lung.
Survival of treated miceFor survival studies, the mice were observed throughout 60 days, and deaths were recorded twice daily. The survival curves of all treated groups are presented in Fig. 6. For C. neoformans infection, AMB-fibrin microsphere was more effective in prolonging survival than AMB-PLGA microsphere, AMB-fibrin beads or free AMB. However, the survival of mice treated with the formulation and AMB-PLGA microsphere were significantly different relative to the untreated controls (p<0.05). AMB-fibrin microsphere based dual therapy increased the survival time of treated mice where almost 80% of the animals survived day 60 post-infection. Treatment with free AMB resulted in survival of 40% of the animals till day 60. AMB-PLGA microsphere and AMB-fibrin beads therapy did also improved the survival rate by 60% and 50%, respectively, as compared to the untreated controls (p<0.05) at day 60 post-infection. In the untreated control group, infection with C. neoformans resulted in 100% mortality by day 20 post-infection.
DiscussionIn spite of the fact that the drug delivery system has been proven to be the most successful medical intervention ever developed, there has been tremendous scope for further improvement to control infectious diseases in human as well as in other mammalian species. The concept of using polymers and blood plasma in a dual delivery system has been conceived because they release the entrapped drug in a sustained manner in the surrounding milieu. After efficiently releasing the drug, these biocompatible and biodegradable polymers break down to lactic acid and glycolic acid that are eventually excreted through the normal process. Plasma, the other component of the system, being part of blood is easily absorbed in the host body upon delivery. So, plasma beads based microspheres will act as a slow release vehicle for delivering the encapsulated drug safely to treat intracellular pathogens. In the present study, the potential of these two components as a vehicle in dual delivery system is documented for therapeutic transport of the antifungal drug AMB against C. neoformans infection in mice.
Fibrin microsphere as the dual delivery system has an exceptional benefit of minimizing the risks of infection and allergic reactions. This is because they are prepared from whole plasma and no extrinsic component is used. Unlike other delivery vehicles, plasma beads are made uniformly with equal dimension and large number of beads can be prepared in a short time. Water insoluble and large molecular weight substances are released from plasma beads by diffusion and subsequently by degradation of fibrin matrix.24,25 Moreover, antifungal drugs possess the ability to bind strongly to the plasma beads26 where entrapped drug is released more slowly from larger beads.18 Likewise, PLGA microsphere is also a well designed drug delivery vehicle that show sustained release of encapsulated drug. In accordance with the above mentioned characteristics, the fibrin microsphere system showed strong entrapment efficiency of about 78% and released AMB slowly in a sustained manner at all tested pH medium (Fig. 2). These parameters support the characteristic potential of AMB-fibrin microsphere as a novel dual delivery system.
After determining the fusogenic potential of AMB entrapped in fibrin microsphere, its antifungal efficacy was tested in experimental murine cryptococcosis. Antifungal efficacy is directly linked to the time of beginning of therapy. It is a general statement that delay in treatment reduces antifungal efficacy.27 Considering this, chemotherapy was started at day 1 post-infection of mice with C. neoformans (1×106cfu/mouse). The dose of AMB used had already been shown to be non-toxic in mice as it did not increase the serum creatinine levels.28
The effects of antifungal therapy were established in terms of fungal burden and survival of treated mice. For determining fungal burden, the decrease in cfu count of C. neoformans was recorded in the systemic circulation, and lung and brain, the two most affected organs. Post chemotherapy, free AMB, AMB-PLGA and AMB-plasma beads were able to reduce the fungal burden but was not comparable with the AMB-fibrin microsphere treated animals. AMB-fibrin microsphere treated animals showed a more pronounced regression of C. neoformans infection as compared to the other delivery systems (Figs. 3–5). The efficacy of chemotherapy by AMB-fibrin microsphere lasted till 21 days. After that no effect was observed on residual fungal burden. The free AMB therapy lasted for only seven days after which no change in fungal burden was recorded at days 14 and 21 (Figs. 3–5). This could be due to clearance of AMB from the infected organs at these times. The effect of AMB is in accordance with already published reports.29,30 Most importantly, the effect of therapy with AMB entrapped in fibrin microsphere persisted for longer time period than other tested forms. Similarly, the survival rate (Fig. 6) was remarkably improved in AMB-fibrin microsphere treated mice as compared to those treated with other formulations. Overall, the effective suppression of the virulence of C. neoformans infection for a longer period demonstrates the superiority of the fibrin microsphere based delivery system.
Consequently, in the present study, the use of plasma beads-PLGA based fibrin microsphere as a delivery vehicle for AMB has been established to play a pivotal role in the clearance of C. neoformans infection in mice. The AMB-fibrin microsphere based therapy showed negligible fungal burden in the analyzed tissues after treatment. Delivery of AMB incorporated in dual delivery system can become a more promising approach than the mono delivery for the treatment of C. neoformans infection. This form of chemotherapy could have an extra advantage over the other traditional drug delivery modalities currently used. The most eye-catching feature is that the fibrin microsphere can be prepared from a host own blood plasma and involves minimum risk in the host upon subsequent exposure that would be required of the concerned person hence further.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this research through the Research Group Project No. RGP-212.