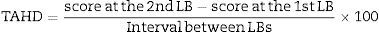To evaluate changes in liver histology in patients with human immunodeficiency virus/hepatitis C virus coinfection non-responders to a suboptimal Interferon+Ribavirine regimen.
Materials and methodsWe investigated 49 patients with two sequential liver biopsies: 18 were non-responders to Interferon+Ribavirine treatment (Group hepatitis C virus Rx) administered after the 1st liver biopsy who underwent a 2nd liver biopsy after a median period of 3.92 year and 31 were patients who remained untreated for hepatitis C virus disease (Group hepatitis C virus untreated) after the 1st liver biopsy because of refusal and underwent a 2nd liver biopsy after a median period of 5.05-years. Most patients in both groups were under highly active antiretroviral therapy. At the time of 1st liver biopsy similar degrees of necro-inflammation, fibrosis and steatosis were observed in both groups. Changes in liver lesions between 1st and 2nd liver biopsys were adjusted for different intervals between liver biopsys by a mathematic formula.
ResultsLiver fibrosis did not change in 88.9% of patients in Group hepatitis C virus Rx and in 77.4% in Group hepatitis C virus untreated. A marked deterioration in liver fibrosis was observed in 5 (16%) patients in Group hepatitis C virus untreated and in none in Group hepatitis C virus treated. Necro-inflammation and steatosis remained substantially unchanged in both groups.
ConclusionLiver histology remained substantially unchanged in human immunodeficiency virus/hepatitis C virus patients non-responder to anti-hepatitis C virus therapy over 4 years observation, suggesting an effective anti-hepatitis C virus early treatment for all hepatitis C virus/human immunodeficiency virus coinfected patients who can reasonably tolerate therapy.
One-third of patients carrying humanimmunodeficiency virus (HIV) have a concomitant hepatitis C virus (HCV) infection1–3 associated with severe liver fibrosis4–6 and unfavorable outcome.3,5–12 More recently, the results of some studies suggest that highly active antiretroviral therapy (HAART), by favoring the re-establishment of the immune-control, may induce a more favorable outcome of HCV related chronic hepatitis.11,3,13–15 Indeed, the methods used to evaluate the progression of liver fibrosis in these studies are somewhat questionable, mostly because they are based on the observation of a single liver biopsy (LB).
We may contribute to this point by analyzing the data from 49 patients with HCV related chronic hepatitis who had undergone two sequential LBs, 18 patients with HIV/HCV coinfection non-responders to an anti-HCV treatment and 31 patients with HIV/HCV coinfection naïve for anti-HCV treatment. The purpose of the present study was to evaluate whether suboptimal anti-HCV treatment that did not achieve sustained virological response (SVR) influenced the progression of liver fibrosis, steatosis and necroinflammation between 1st and 2nd LB.
Materials and methodsOut of 440 HIV infected patients with chronic hepatitis C who underwent liver biopsy from 1995 to 2007 we selected 49 patients with chronic hepatitis C who had undergone two sequential LBs at an interval of at least 2 years.
Of these 49 HIV/HCV coinfected patients with chronic hepatitis, 18 were non-responders to treatment with Interferon (INF) or INF plus Ribavirine after the 1st LB (Group HCV Rx); eight had HCV genotype 1 and 10 had HCV genotype 3. In relation to the period of time they were investigated, nine patients in this group received standard INF α2a/α2b 6 MU TIW, associated with Ribavirine 800–1000mg daily in four patients, and nine Peg-INF α2a/α2b one injection weekly, associated with Ribavirine 800–1000mg daily in seven patients. Fifteen (83.4%) of these 18 patients were under HAART during the study (Table 1). All patients discontinued treatment after 3–4 months due to lack of response according to current international guidelines. These 18 patients underwent a 2nd LB to assess possible changes in liver histology at the time (median 5.05 years) they were re-evaluated for re-treatment.
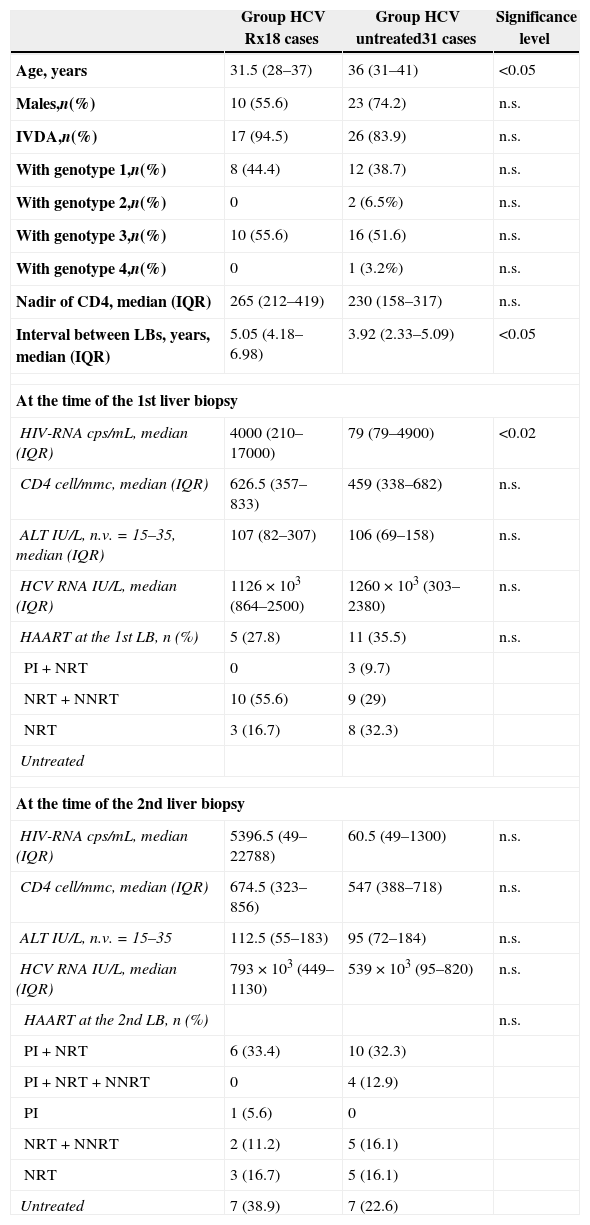
Comparison of demographic, epidemiological and immuno-virological data at the time of 1st and 2nd liver biopsy (LB) between Group HCV Rx and Group HCV untreated.
| Group HCV Rx18 cases | Group HCV untreated31 cases | Significance level | |
|---|---|---|---|
| Age, years | 31.5 (28–37) | 36 (31–41) | <0.05 |
| Males,n(%) | 10 (55.6) | 23 (74.2) | n.s. |
| IVDA,n(%) | 17 (94.5) | 26 (83.9) | n.s. |
| With genotype 1,n(%) | 8 (44.4) | 12 (38.7) | n.s. |
| With genotype 2,n(%) | 0 | 2 (6.5%) | n.s. |
| With genotype 3,n(%) | 10 (55.6) | 16 (51.6) | n.s. |
| With genotype 4,n(%) | 0 | 1 (3.2%) | n.s. |
| Nadir of CD4, median (IQR) | 265 (212–419) | 230 (158–317) | n.s. |
| Interval between LBs, years, median (IQR) | 5.05 (4.18–6.98) | 3.92 (2.33–5.09) | <0.05 |
| At the time of the 1st liver biopsy | |||
| HIV-RNA cps/mL, median (IQR) | 4000 (210–17000) | 79 (79–4900) | <0.02 |
| CD4 cell/mmc, median (IQR) | 626.5 (357–833) | 459 (338–682) | n.s. |
| ALT IU/L, n.v.=15–35, median (IQR) | 107 (82–307) | 106 (69–158) | n.s. |
| HCV RNA IU/L, median (IQR) | 1126×103 (864–2500) | 1260×103 (303–2380) | n.s. |
| HAART at the 1st LB, n (%) | 5 (27.8) | 11 (35.5) | n.s. |
| PI+NRT | 0 | 3 (9.7) | |
| NRT+NNRT | 10 (55.6) | 9 (29) | |
| NRT | 3 (16.7) | 8 (32.3) | |
| Untreated | |||
| At the time of the 2nd liver biopsy | |||
| HIV-RNA cps/mL, median (IQR) | 5396.5 (49–22788) | 60.5 (49–1300) | n.s. |
| CD4 cell/mmc, median (IQR) | 674.5 (323–856) | 547 (388–718) | n.s. |
| ALT IU/L, n.v.=15–35 | 112.5 (55–183) | 95 (72–184) | n.s. |
| HCV RNA IU/L, median (IQR) | 793×103 (449–1130) | 539×103 (95–820) | n.s. |
| HAART at the 2nd LB, n (%) | n.s. | ||
| PI+NRT | 6 (33.4) | 10 (32.3) | |
| PI+NRT+NNRT | 0 | 4 (12.9) | |
| PI | 1 (5.6) | 0 | |
| NRT+NNRT | 2 (11.2) | 5 (16.1) | |
| NRT | 3 (16.7) | 5 (16.1) | |
| Untreated | 7 (38.9) | 7 (22.6) | |
The remaining 31 patients with HIV/HCV coinfection and chronic hepatitis declined treatment for HCV infection after the 1st LB (Group HCV untreated) and were followed as well. Patients in this group underwent a 2nd LB for a new assessment of liver histology at the time (median 3.9 years) they accepted to be re-evaluated for anti-HCV treatment. Twenty-four (77.4%) patients in this group received HAART throughout the study (Table 1).
Patients were enrolled at the Clinic of Infectious Diseases of the San Raffaele Scientific Institute in Milan and investigated in cooperation with the Clinic of Infectious Diseases of the Second University of Naples. The procedures followed in this study were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983, and each patient signed an informed consent before each LB.
For patients in both groups, the data recorded at the baseline (time of 1st LB) and at the time of 2nd LB included risk factors for acquiring HIV and HCV infection, presumed time of HIV and HCV infection, liver function tests, CD4+ and CD8+ counts, HCV-genotype, HIV and HCV viral loads, and routine tests. The patients were observed at a three-month interval with physical examination and laboratory tests exploring liver and kidney functions, blood cell count, CD4+ cell count, HIV and HCV viral loads.
Excluded from this study were patients who had been treated with α-INF or Peg-INF, alone or in combination with Ribavirin before the 1st LB, those with a history of alcohol abuse (>40g/day for males and >30g/day for females for at least 5 years), those with HBsAg positive or autoimmune hepatitis, and those with genetic disorders possibly inducing liver disease.
Liver histologyLB was performed percutaneously under US guidance; specimens were fixed in formalin, embedded in paraffin and stained with hematoxylin–eosin and Masson's trichrome stains. The histological data were analyzed by a pathologist who was unaware of the clinical and laboratory data using Ishak's scoring system for HAI and fibrosis16 and a home made scoring system for steatosis he had been using for decades (score 1=1–10% of hepatocytes with fatty deposition, score 2=11–30%; score 3=31–60%; score 4≥60%).
To compare the two groups of patients for changes in fibrosis, necro-inflammation and steatosis, avoiding the bias due to the different interval between LBs, a time-adjusted histological difference (TAHD) was calculated for each histological lesion and in each single LB:
A negative TAHD indicated an improvement and a positive TAHD indicated deterioration.For an easier reading of the data, the TAHD value was converted into Arbitrary Units, named Improvement Progression Unit (IPU) for negative TAHD or Deterioration Progression Unit (DPU) for positive TAHD, where one Unit represents the standard difference of one degree of a histological lesion in the four years median interval between 2nd and 1st LB: one Progression Unit (IPU or DPU)=one degree difference/four years×100=25 TAHD points. On the basis of this formula, we considered as improved all patients with a negative TADH equivalent to two or more IPUs: an improvement of 2–6 IPUs was considered as moderate and an improvement of more than 6 IPUs as marked. Conversely, all patients with a positive TAHD equivalent to two or more DPUs were considered to have deteriorated: a deterioration of 2–6 DPUs was considered as moderate and a deterioration of more than six DPUs as marked. The histological lesions were considered unchanged when variations in the degree of necro-inflammation, fibrosis or steatosis between the 2nd and 1st LB were minimal (<2 IPUs, or <2 DPUs) or absent.
Laboratory testsAntibody to HCV was determined by a 3rd generation commercial immunoenzymatic assay. HCV RNA was determined in plasma samples by a qualitative retro-transcriptase PCR with primers for 5′ non-coding region of the viral genome, using a commercial kit with the lowest detection limit of 200 copies. In HCV-RNA positive cases, HCV RNA was assessed using quantitative retro-transcriptase PCR (HCV-Amplicor Monitor 2.0; Roche Molecular System, Branchburg, NJ, USA), which shows the lowest detection limit of 600IU/mL.
HCV genotyping was performed by a line-probe assay (INNO-LIPA HCV-II; Innogenetics, Zwijndrecht, Belgium).
Antibodies to HIV-1 and 2 were determined by commercial ELISA and positive results were confirmed by Western blot, which identifies antibodies to the HIV-1 and HIV-2 strains.
HIV viral load was assessed using the Amplicor HIV Monitor 1 test (Roche Molecular Systems Inc., Branchburg, NJ, USA) with the lowest detection limit of 400copies/mL.
HBsAg, anti-HBs and total anti-HBc were tested using commercial immunoenzymatic assays. Lymphocyte subsets (CD4+, CD8+) were evaluated by flow cytofluorimetry using monoclonal antibodies and a fluorescence-activated cell sorter scan (Becton Dickinson, Mountain View, USA). Liver function tests were carried out according to routine methods.
Statistical analysisThe analyses were performed using SAS software (version 8.2; SAS Institute, Cary, NC, USA). All tests of significance were 2-sided, and p<0.05 was considered statistically significant. Some continuous parameters were stratified according to their median values and then used and compared as categorical variables. Associations between discrete variables were tested by χ2 or Fisher's exact test, as appropriate.
ResultsAt the time of the 1st LB patients in Group HCV Rx compared with those in Group HCV untreated were younger (p<0.05) and with higher HIV viral load (p<0.02) (Table 1). No other demographic, epidemiological and immuno-virological difference was found between these two groups, neither at the time of the 1st nor at the 2nd LB. Also the HAART regimen was similar in these two groups (Table 1).
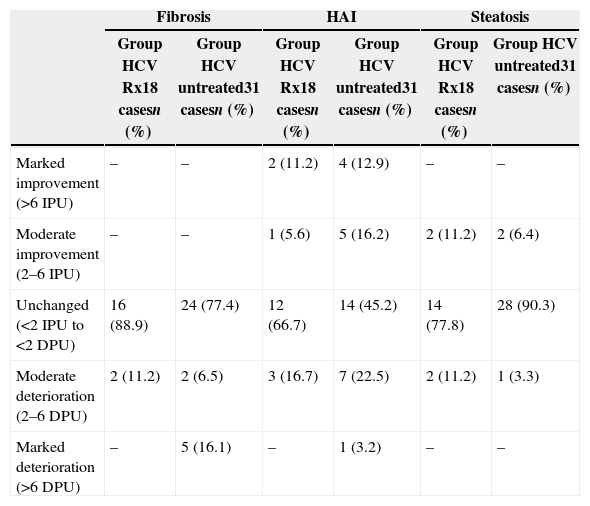
The raw histological scores observed in both groups are reported in Table 2 for an analytical evaluation, whereas the differences in necro-inflammation, fibrosis and steatosis are presented as IPU and DPU in Table 3. Liver fibrosis remained substantially unchanged in 16 (88.9%) of the 18 patients in Group HCV Rx, whereas a moderate deterioration was observed only in the remaining two (11.1%) (Table 3). Instead, of the 31 patients in Group HCV untreated, 24 (77.4%) remained substantially unchanged, two (6.5%) showed a moderate deterioration and five (16.1%) a marked deterioration in liver fibrosis (Table 3). These differences are not statistically significant, but the data are of clinical relevance since all the seven patients who deteriorated in Group HCV untreated had the minimal score of fibrosis (score 1) at the first LB, whereas in the 2nd LB, 3.6 years later (median value in these seven patients), one patient progressed to score 3, another one to score 4 and five to liver cirrhosis (two with score 5 and three to score 6).
Scores of liver lesions (fibrosis, necro-inflammation (HAI) and steatosis) in the 1st and 2nd liver biopsy (LB) in Group HCV Rx and Group HCV untreated.
| Group HCV Rx18 cases | Group HCV untreated31 cases | |
|---|---|---|
| Degree of fibrosis | ||
| 1st LB, n (%) | ||
| 0 | 1 (5.6) | 0 |
| 1 | 7 (38.9) | 16 (51.6) |
| 2 | 3 (16.7) | 8 (25.8) |
| 3 | 4 (22.3) | 1 (3.2) |
| 4 | 3 (16.7) | 1 (3.2) |
| 5 | 0 | 3 (9.7) |
| 6 | 0 | 2 (6.5) |
| 2nd LB, n (%) | ||
| 0 | 0 | 1 (3.2) |
| 1 | 4 (22.3) | 10 (32.3) |
| 2 | 10 (55.6) | 5 (16.1) |
| 3 | 2 (11.2) | 3 (9.7) |
| 4 | 2 (11.2) | 1 (3.2) |
| 5 | 0 | 5 (16.1) |
| 6 | 0 | 6 (19.4) |
| Degree of necro-inflammation | ||
| 1st LB, n (%) | ||
| 0 | 0 | 2 (6.5) |
| 1–3 | 12 (66.7) | 13 (41.9) |
| 4–8 | 4 (22.3) | 12 (38.7) |
| 9–12 | 0 | 1 (3.2) |
| 13–18 | 2 (11.2) | 3 (9.7) |
| 2nd LB, n (%) | ||
| 0 | 0 | 4 (12.9) |
| 1–3 | 8 (44.5) | 12 (38.7) |
| 4–8 | 9 (50) | 12 (38.7) |
| 9–12 | 1 (5.6) | 3 (9.7) |
| 13–18 | 0 | 0 |
| Degree of steatosis | ||
| 1st LB, n (%) | ||
| 0 | 6 (33.4) | 7 (22.6) |
| 1 | 4 (22.3) | 11 (35.5) |
| 2 | 0 | 1 (3.2) |
| 3 | 5 (27.8) | 5 (16.1) |
| 4 | 3 (16.7) | 7 (19.4) |
| 2nd LB, n (%) | ||
| 0 | 7 (38.9) | 10 (32.3) |
| 1 | 7 (38.9) | 9 (29) |
| 2 | 0 | 0 |
| 3 | 1 (38.9) | 4 (12.9) |
| 4 | 3 (16.7) | 8 (25.8) |
Histological changes between the 1st and 2nd liver biopsy (LB) in Group HCV Rx and Group HCV untreated, expressed as Improvement Progression Unit (IPU) or Deterioration Progression Unit (DPU) for liver fibrosis, necro-inflammation (HAI) and steatosis.
| Fibrosis | HAI | Steatosis | ||||
|---|---|---|---|---|---|---|
| Group HCV Rx18 casesn (%) | Group HCV untreated31 casesn (%) | Group HCV Rx18 casesn (%) | Group HCV untreated31 casesn (%) | Group HCV Rx18 casesn (%) | Group HCV untreated31 casesn (%) | |
| Marked improvement (>6 IPU) | – | – | 2 (11.2) | 4 (12.9) | – | – |
| Moderate improvement (2–6 IPU) | – | – | 1 (5.6) | 5 (16.2) | 2 (11.2) | 2 (6.4) |
| Unchanged (<2 IPU to <2 DPU) | 16 (88.9) | 24 (77.4) | 12 (66.7) | 14 (45.2) | 14 (77.8) | 28 (90.3) |
| Moderate deterioration (2–6 DPU) | 2 (11.2) | 2 (6.5) | 3 (16.7) | 7 (22.5) | 2 (11.2) | 1 (3.3) |
| Marked deterioration (>6 DPU) | – | 5 (16.1) | – | 1 (3.2) | – | – |
Changes in necro-inflammation scores were quite frequent but similar in both groups (Table 3).
Changes in liver steatosis were infrequent in both groups and differences were not statistically significant (Table 3).
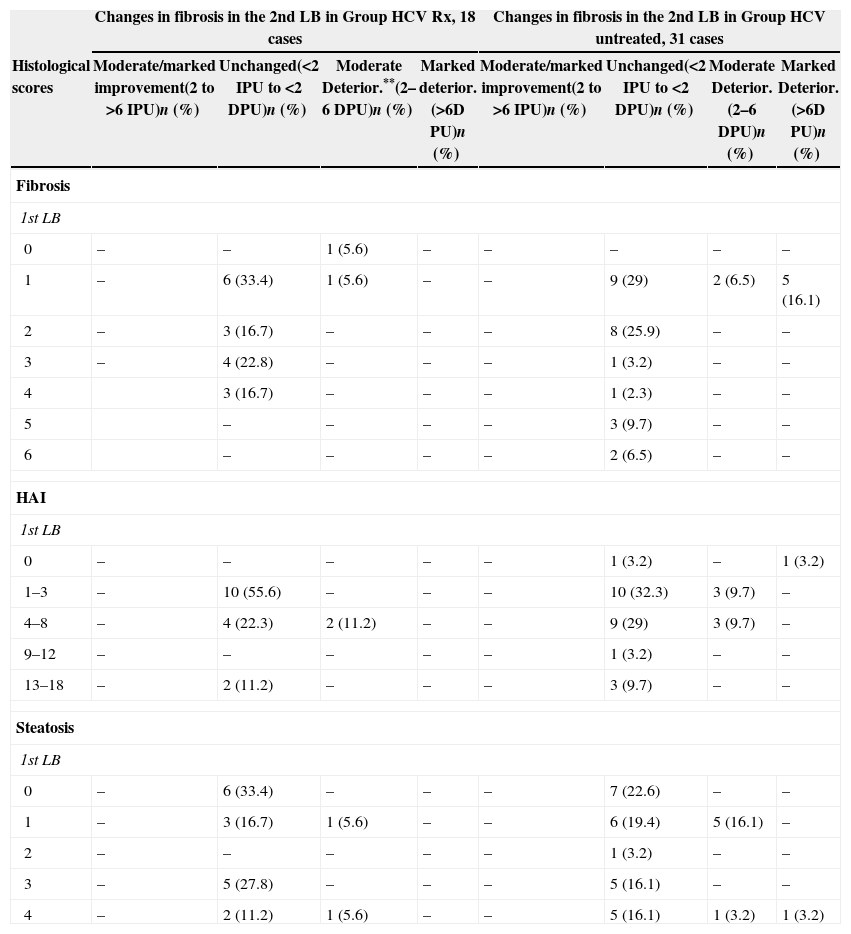
In both groups no association was found between the degree of liver fibrosis, necro-inflammation or liver steatosis in the 1st LB and a peculiar progression of these lesions in the 2nd LB. Nevertheless, the data regarding liver fibrosis are reported in Table 4, because of the relevance of this lesion on the course of chronic hepatitis and of the contrasting data on the possibility to predict the progression to fibrosis reported in literature.6,20,21,23–26Table 4 shows, in terms of IPU or DPU, no correlation between the degrees of liver fibrosis, necro-inflammation and steatosis observed in the 1st LB and changes in liver fibrosis found in the 2nd LB.
Correlation between liver fibrosis, necroinflammation (HAI) or steatosis scores in the 1st Liver biopsy (LB) and changes in liver fibrosis in the 2nd LB, expressed as Improvement Progression Unit (IPU) or Deterioration Progression Unit (DPU) in Group HCV Rx and Group HCV untreated.
| Changes in fibrosis in the 2nd LB in Group HCV Rx, 18 cases | Changes in fibrosis in the 2nd LB in Group HCV untreated, 31 cases | |||||||
|---|---|---|---|---|---|---|---|---|
| Histological scores | Moderate/marked improvement(2 to >6 IPU)n (%) | Unchanged(<2 IPU to <2 DPU)n (%) | Moderate Deterior.**(2–6 DPU)n (%) | Marked deterior.(>6D PU)n (%) | Moderate/marked improvement(2 to >6 IPU)n (%) | Unchanged(<2 IPU to <2 DPU)n (%) | Moderate Deterior.(2–6 DPU)n (%) | Marked Deterior.(>6D PU)n (%) |
| Fibrosis | ||||||||
| 1st LB | ||||||||
| 0 | – | – | 1 (5.6) | – | – | – | – | – |
| 1 | – | 6 (33.4) | 1 (5.6) | – | – | 9 (29) | 2 (6.5) | 5 (16.1) |
| 2 | – | 3 (16.7) | – | – | – | 8 (25.9) | – | – |
| 3 | – | 4 (22.8) | – | – | – | 1 (3.2) | – | – |
| 4 | 3 (16.7) | – | – | – | 1 (2.3) | – | – | |
| 5 | – | – | – | – | 3 (9.7) | – | – | |
| 6 | – | – | – | – | 2 (6.5) | – | – | |
| HAI | ||||||||
| 1st LB | ||||||||
| 0 | – | – | – | – | – | 1 (3.2) | – | 1 (3.2) |
| 1–3 | – | 10 (55.6) | – | – | – | 10 (32.3) | 3 (9.7) | – |
| 4–8 | – | 4 (22.3) | 2 (11.2) | – | – | 9 (29) | 3 (9.7) | – |
| 9–12 | – | – | – | – | – | 1 (3.2) | – | – |
| 13–18 | – | 2 (11.2) | – | – | – | 3 (9.7) | – | – |
| Steatosis | ||||||||
| 1st LB | ||||||||
| 0 | – | 6 (33.4) | – | – | – | 7 (22.6) | – | – |
| 1 | – | 3 (16.7) | 1 (5.6) | – | – | 6 (19.4) | 5 (16.1) | – |
| 2 | – | – | – | – | – | 1 (3.2) | – | – |
| 3 | – | 5 (27.8) | – | – | – | 5 (16.1) | – | – |
| 4 | – | 2 (11.2) | 1 (5.6) | – | – | 5 (16.1) | 1 (3.2) | 1 (3.2) |
Apparently, non-effective anti-HCV treatment was of some benefit to our HIV/HCV coinfected patients with chronic hepatitis. In fact, no substantial deterioration in liver histology was observed after a median observation period of 5 years in 18 non-responder patients to anti-HCV treatment, whereas nearly a quarter of the untreated patients progressed from the minimal degree of fibrosis to moderate fibrosis (6.5%) or to liver cirrhosis (16.1%) in four years. Lack of progression to a more severe liver fibrosis was also reported by Chung et al. in one-third of 66 HIV/HCV coinfected patients who did not achieve SVR following anti-HCV treatment.17
Independent predictors of fibrosis progression in HIV/HCV coinfection have been suggested, such as an old age at the time of HCV infection, alcohol intake, and the entity of the necro-inflammatory lesions.18 However, due to the different structure of published studies it is difficult to reach a definitive conclusion on this point. The HIV/HCV confected patients in the present investigation did not show such cofactors for fibrosis progression since they had acquired HCV infection at a young age, most of them showed a low score of necro-inflammation and none of them admitted a history of alcohol abuse. Moreover, most of them received an optimized HAART regimen.
Some authors found HCV-genotype 3 associated with liver steatosis in HIV/HCV coinfected patients,19–24 an association denied by other investigators.25 Liver steatosis was considered a risk factor for developing a more severe fibrosis in HIV/HCV coinfected patients,22,24,26 a datum not confirmed by other authors6,27 and by the results of the present study showing in both groups no correlation between the initial score of liver steatosis and changes in fibrosis in the 2nd LB.
Indeed, no correlation was also found in both groups of patients between the score of necro-inflammation in the 1st LB and the progression of this lesion, liver fibrosis and liver steatosis nor between the score of liver fibrosis in the 1st LB and the progression of liver fibrosis itself, steatosis and necro-inflammation in the 2nd one. These data allow to concluding that the evolution of liver fibrosis, necro-inflammation and steatosis in patients with HIV/HCV coinfection may hardly be predicted by the initial degree of these histological lesions.
Concluding, the data of the present study indicate that the progression to a more severe stage of liver fibrosis or to cirrhosis observed in nearly a quarter of untreated patients over a period of four years may possibly be prevented by an anti-HCV treatment. This strongly suggests to consider early anti-HCV treatment, possibly including a newly developed protease inhibitor, all HIV/HCV coinfected patients who can reasonably tolerate therapy.
Conflicts of interestThe authors declare no conflicts of interest.