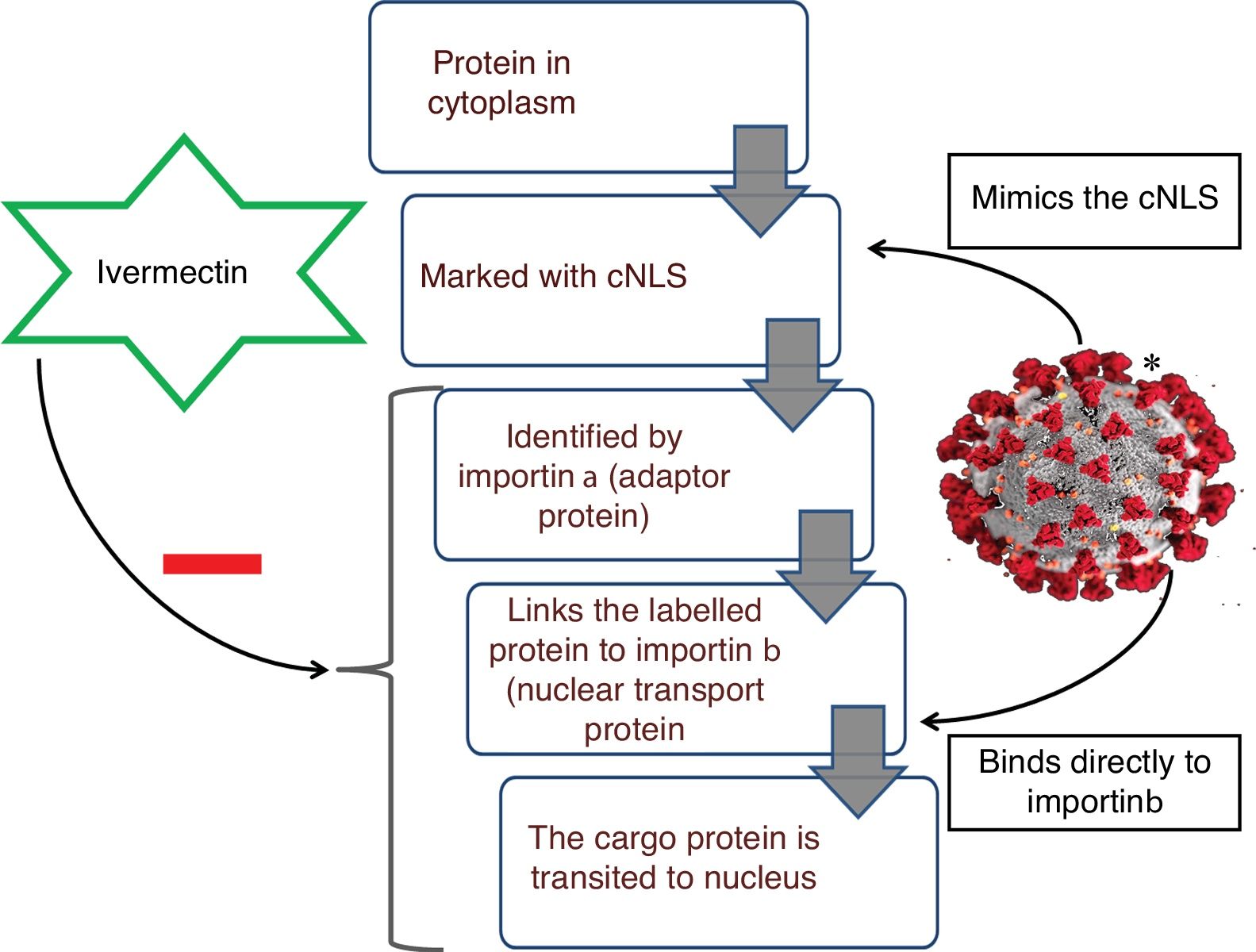
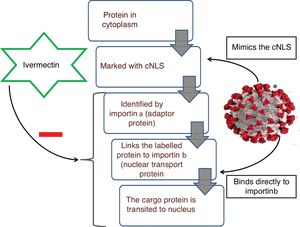
Ivermectin, a well-known anti-helmintic agent from the late-1970s, causes stimulation of gamma amino butyric acid (GABA)-gated-Cl− channels, leading to hyperpolarization, and resulting in paralysis of the infesting organism. Another mechanism that has been postulated for the same effect is the immunomodulation of host response. This is attained by the activation of neutrophils, increase in the levels of C-reactive protein and interleukin-6.1 In recent times, the antiviral function of ivermectin has been discovered, which appears to be intriguing. Already its effectiveness against certain flavivirus (dengue fever, Japanese encephalitis and tick-borne encephalitis virus) and chikungunya virus has been demonstrated in vitro.2,3 Since then the same activity has been assessed in numerous other viral infections. Off lately its potency has been recognized in eliminating coronavirus in vitro. The exact mechanism to which this effect can be attributed to is yet to be validated, but the speculated method is inhibition of importin α/β1 mediated transport of viral proteins in and out of the nucleus.4 Importins, a type of karyopherins, exemplify a major class of soluble transport receptors which are involved in nucleo-cytoplasmic transit of various substrates (Fig. 1).5 The speculated inhibitory action of ivermectin on importin α/β mediated transport system, Based on this conjecture, the role of ivermectin in eliminating Covid-19 can be assumed.
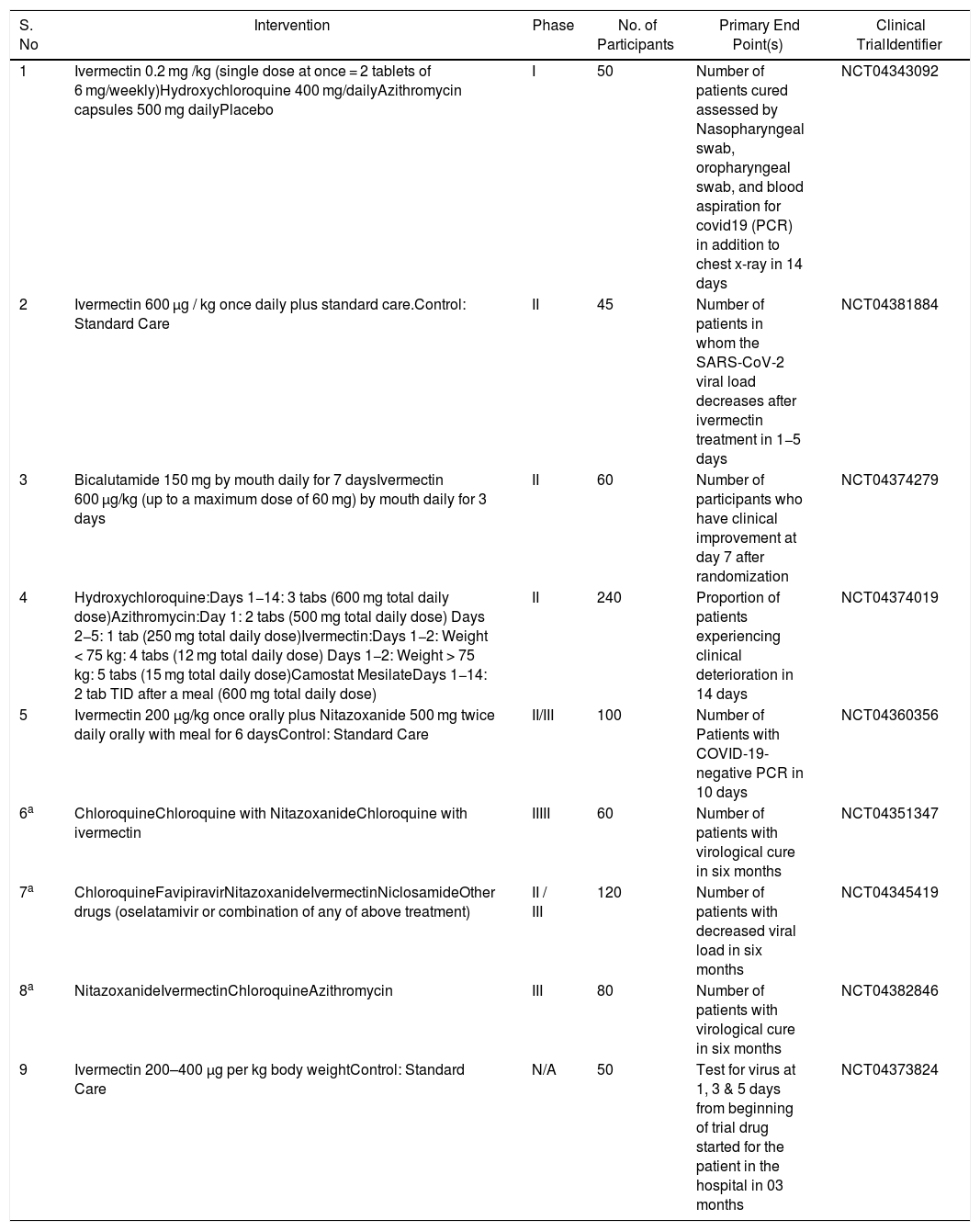
Until now, in only single in vitro study, the efficacy of ivermectin against coronavirus has been demonstrated. Caly et al. tested for the viral RNA levels in both supernatant and cell pellets of the Vero/hSLAM cells which were infected with SARS-CoV-2 (isolate Australia/VIC01/2020), and were then treated with 5 μM ivermectin two hours later. After 24 h, they observed a decline of about 93% and 98% in viral RNA levels and cell-associated viral RNA, respectively. Later at 48 h, they detected further reduction (∼5000 fold) in the viral RNA load only. To ascertain this finding, the infected cells were treated with serial dilutions of ivermectin, and were then tested for viral RNA load by RT-PCR. With this research, the investigators could comment about the inhibitory concentration 50 (IC50) which was estimated to be ∼2 μM, and also that no toxicities were noticed for the various concentrations at which ivermectin was tested.6 Based on the efficacy evidenced in in vitro study, various clinical studies have been planned and started, though none of them have yet been completed (Table 1).
Salient features of ongoing clinical trials of ivermectin for COVID-19.
| S. No | Intervention | Phase | No. of Participants | Primary End Point(s) | Clinical TrialIdentifier |
|---|---|---|---|---|---|
| 1 | Ivermectin 0.2 mg /kg (single dose at once = 2 tablets of 6 mg/weekly)Hydroxychloroquine 400 mg/dailyAzithromycin capsules 500 mg dailyPlacebo | I | 50 | Number of patients cured assessed by Nasopharyngeal swab, oropharyngeal swab, and blood aspiration for covid19 (PCR) in addition to chest x-ray in 14 days | NCT04343092 |
| 2 | Ivermectin 600 µg / kg once daily plus standard care.Control: Standard Care | II | 45 | Number of patients in whom the SARS-CoV-2 viral load decreases after ivermectin treatment in 1−5 days | NCT04381884 |
| 3 | Bicalutamide 150 mg by mouth daily for 7 daysIvermectin 600 µg/kg (up to a maximum dose of 60 mg) by mouth daily for 3 days | II | 60 | Number of participants who have clinical improvement at day 7 after randomization | NCT04374279 |
| 4 | Hydroxychloroquine:Days 1−14: 3 tabs (600 mg total daily dose)Azithromycin:Day 1: 2 tabs (500 mg total daily dose) Days 2−5: 1 tab (250 mg total daily dose)Ivermectin:Days 1−2: Weight < 75 kg: 4 tabs (12 mg total daily dose) Days 1−2: Weight > 75 kg: 5 tabs (15 mg total daily dose)Camostat MesilateDays 1−14: 2 tab TID after a meal (600 mg total daily dose) | II | 240 | Proportion of patients experiencing clinical deterioration in 14 days | NCT04374019 |
| 5 | Ivermectin 200 μg/kg once orally plus Nitazoxanide 500 mg twice daily orally with meal for 6 daysControl: Standard Care | II/III | 100 | Number of Patients with COVID-19-negative PCR in 10 days | NCT04360356 |
| 6a | ChloroquineChloroquine with NitazoxanideChloroquine with ivermectin | IIIII | 60 | Number of patients with virological cure in six months | NCT04351347 |
| 7a | ChloroquineFavipiravirNitazoxanideIvermectinNiclosamideOther drugs (oselatamivir or combination of any of above treatment) | II / III | 120 | Number of patients with decreased viral load in six months | NCT04345419 |
| 8a | NitazoxanideIvermectinChloroquineAzithromycin | III | 80 | Number of patients with virological cure in six months | NCT04382846 |
| 9 | Ivermectin 200–400 μg per kg body weightControl: Standard Care | N/A | 50 | Test for virus at 1, 3 & 5 days from beginning of trial drug started for the patient in the hospital in 03 months | NCT04373824 |
All the details mentioned, have been obtained from https://clinicaltrials.gov/.
The in vitro potency of ivermectin against Covid-19 virus is a testimony that this drug can be utilized to manage those patients who have been infected with SARS-CoV-2. Since the conditions in which the virus replicates and infects the cells in vivo and in vitro differs, a decisive comment about how ivermectin may prove to be beneficial to the patients cannot be constructed yet. Similarly, any disparity in the pharmacokinetic properties of this drug and the unidentified drug interactions which may occur under such conditions are yet to be recognized and remarked on. Nevertheless if compared with the other pharmacotherapeutic options for the management of Covid-19 infection, ivermectin may prove to have leverage over them. In addition to a different mechanism of action, there are other facets as well in which this drug may have an upper hand. For instance, the adverse effects associated with hydroxychloroquine (irreversible retinal damage, prolong QT interval, myopathy, neuropathy) or with lopinavir + ritonavir combination (hypertriglyceridemia, hypercholesterolemia) are not seen in patients who are on ivermectin. Furthermore, the treatment regimen with ivermectin may turn out to be more cost-effective. The therapeutic regimen with hydroxychloroquine and azithromycin combination comes out to be ∼5−6 times more expensive than the one with ivermectin. The same can be commented about the patent antivirals which are priced at exorbitant rates. Another worthwhile issue to be addressed is the over-utilization of hydroxychloroquine in managing the Covid-19 patients, may create an apparent shortage of this drug which is a standard treatment for patients with auto-immune diseases.
Taking into account these lacunae and merits, it becomes imperative that clinical trials with ivermectin be conducted in patients of Covid-19, to comprehend whether this drug can provide beneficial effect to those patients who have already developed complications due to this infection.
FundingNo funding received.
Conflict of interestThe authors declare no conflicts of interest.