Prosthetic Vascular Graft Infection (PVGI) is a rare but serious complication of open surgical repair of abdominal aortic aneurysm. We report a case of gas-forming abscesses associated with PVGI caused by Bacteroides fragilis identified from the drainage fluid and blood cultures.
Case presentationA 67-year-old man with a complaint of worsening low back pain underwent an emergency open surgery due to abdominal aortic aneurysm. On postoperative day 18, an abdominal CT showed gas formation within the aneurysm. Gram staining of the drainage fluid revealed Gram-negative rods and 16S rRNA gene sequencing performed on the fluid identified B. fragilis, although the culture of the fluid was negative. PVGI caused by anaerobic bacteria such as Bacteroides spp. is relatively rare. The sensitivity of 16S rRNA gene sequencing for culture-negative specimens is generally low, but 16S rRNA sequencing-positive rate increases when a Gram stain of specimen is positive.
Prosthetic Vascular Graft Infection (PVGI) has been reported to be a complication of open surgical repair of abdominal aortic aneurysm in 0.6 % to 3 % of the cases.1 Although Gram-positive cocci are the most common pathogens of PVGI, anaerobic bacteria have also been implicated.2 Because PVGI requires long-term antibiotic therapy, identification of the causative organism is important for appropriate antibiotic use. However, in the clinical setting, the causative organism often cannot be identified because of the difficulty in obtaining appropriate specimens, resulting in the need to administer empirical broad-spectrum antibiotics for a prolonged period.
We report a case of PVGI following open surgical repair of abdominal aortic aneurysm, in which culture of drainage fluid collected under antibiotic treatment was negative, but 16 s rRNA sequencing identified the causative organism, allowing selection of appropriate antibiotic regimen.
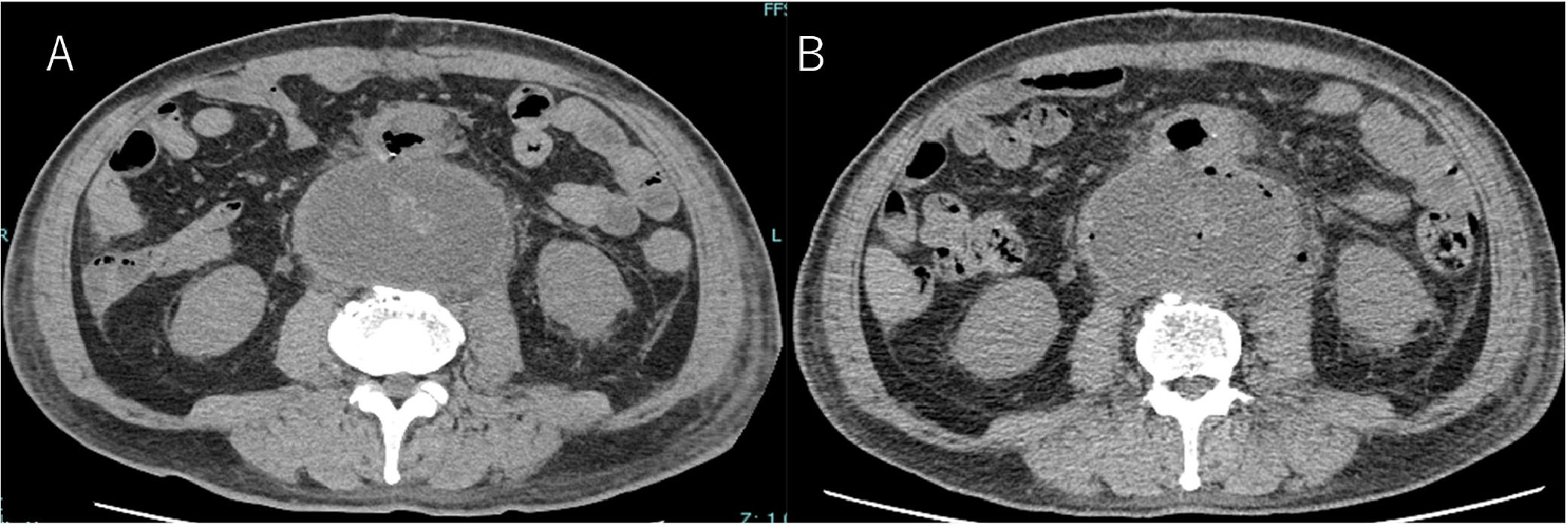
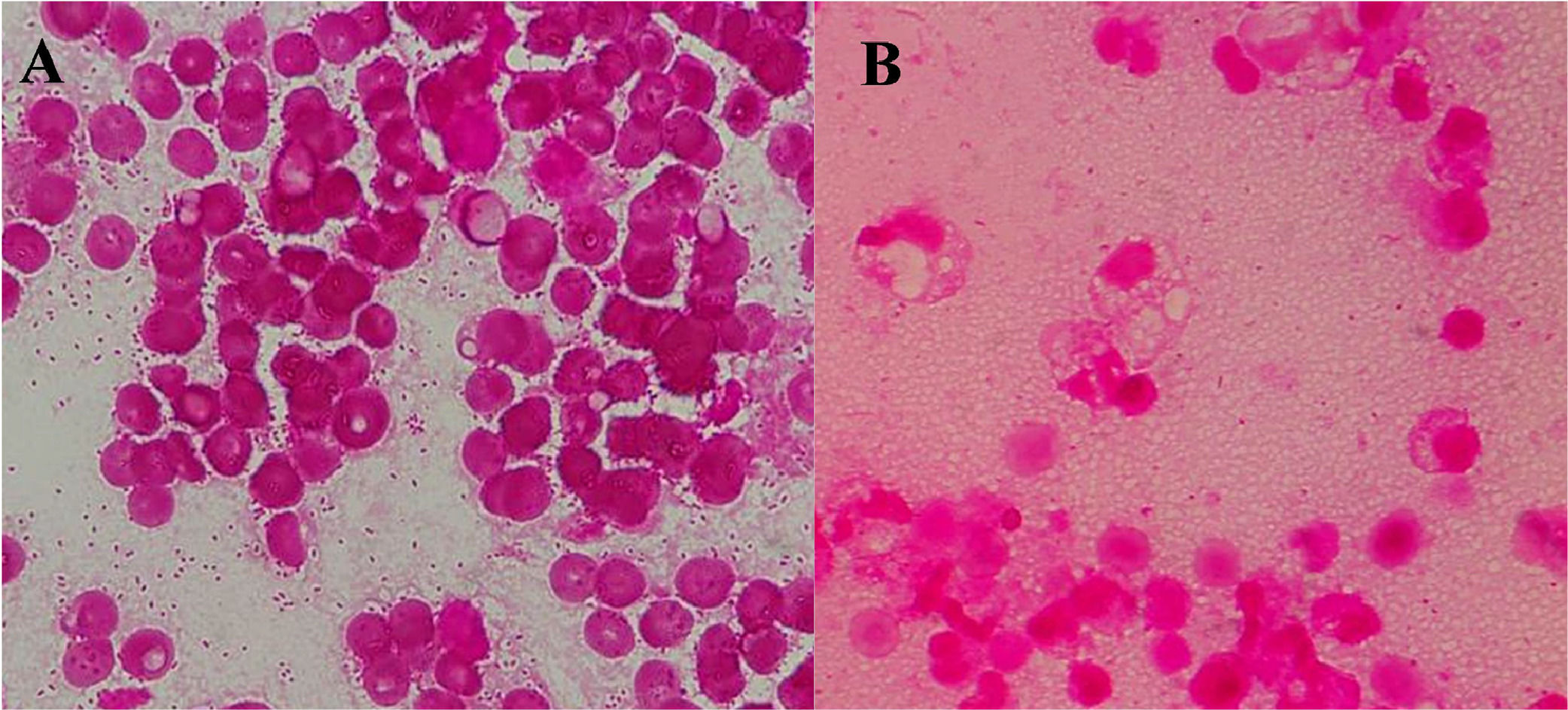
Case presentationA 67-year-old man with a history of hypertension, hyperuricemia, and chronic kidney disease was admitted to our hospital with a complaint of worsening low back pain for a week. An abdominal contrast-enhanced Computed Tomography (CT) scan revealed an abdominal aortic aneurysm with maximum minor-axis diameter of 120 mm, and rupture of the inferior vena cava. On admission, an open emergency surgery for aorta replacement was performed. On day 11, his body temperature exceeded 38 °C. Laboratory tests indicated normal White Blood Cell (WBC) count (6820 μL) and slightly elevated C-Reactive Protein (CRP) level (1.22 mg/dL). After blood cultures were collected, meropenem was empirically administered. On day 12, an abdominal CT scan showed a reduced abdominal aortic aneurysm (maximum minor-axis diameter of 72 mm) (Fig. 1A). On day 13, two sets of blood cultures yielded Gram-negative rods (Fig. 2A), which were later identified as Bacteroides fragilis by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonics, https://www.bruker.com) (score value, 2.208). The minimum inhibitory concentrations of the organisms measured by a broth microdilution method were as follows: piperacillin, 64 µg/mL; cefmetazole, 2 µg/mL; cefotaxime, > 64 µg/mL; piperacillin-tazobactam, ≤ 1 µg/mL; meropenem, ≤ 0.5 µg/mL; ciprofloxacin, 64 µg/mL; minocycline, 1 µg/mL; and metronidazole, ≤ 0.5 µg/mL. After starting antibiotics, his condition tended to improve. However, on day 18, he presented to the infectious disease consultation with a recurrent fever. His vital signs were as follows: body temperature 38.4 °C, blood pressure 118/68 mmHg, heart rate 82 beats/min, respiratory rate 15 breaths/min, and oxygen saturation of 96 % (room air). The stool was loose. Other physical examination findings were unremarkable. Laboratory data at the time of consultation were as follows: WBC 9890 μL (neutrophils 79.0 %, lymphocytes 12.8 %, monocytes 6.4 %, eosinophils 1.5 %, basophils 0.3 %), hemoglobin 7.4 g/dL, hematocrit 22 %, platelets 20.0 × 106/L, serum creatinine 1.43 mg/dL, CRP 18.4 mg/dL, and PCT 1.6 ng/dL. Echocardiography showed no vegetation, while an abdominal CT showed gas formation within the aneurysm and no evidence of aorta-enteric fistula (Fig. 1B). Based on the findings, A gas-forming abscess associated with PVGI caused by B. fragilis was suspected. Considering the possibility of drug fever, the antibiotic regimen was changed to ceftriaxone and metronidazole. On day 21, additional irrigation drainage and omental flap coverage were performed to control infection, followed by blood and drainage fluid sampling. Blood cultures were negative. Gram stain of drainage fluid sample revealed the presence of Gram-negative rods (Fig. 2B), but the culture of the fluid was negative. The drainage fluid sample was analyzed by 16S rRNA gene sequencing, which identified B. fragilis with 99.8 % nucleotide identity (1473 of 1476 nucleotides) to the B. fragilis strain CCUG4856T (GenBank accession number: CP036555.1). The final diagnosis was gas-forming abscess associated with PVGI caused by B. fragilis. Consequently, we recommended discontinuation of ceftriaxone based on the diagnosis. However, combination therapy with ceftriaxone and metronidazole was continued because cardiac surgeons were concerned about possible other infections. Antimicrobial therapy was discontinued on day 47, and the patient was discharged on day 53. He has had an uneventful post-discharge course without recurrence.
The most common pathogens isolated in PVGI are Staphylococcus aureus and coagulase-negative staphylococci.2 PVGI caused by anaerobic bacteria such as Bacteroides spp. is relatively rare.2 Gas production in the vessels is suggestive of PVGI.2 On the other hand, peri‑graft gas generally resolves within 1-week after operation but may persist for up to 7 weeks after open surgery.3 In our case, on postoperative day 11, an abdominal CT scan showed no gas production. Therefore, we considered a low possibility of gas formation after operation.
Gas formation is a result of mixed acid fermentation of glucose by enzymes produced by some organisms such as Enterobacterales.4 Although Clostridium perfringens, Clostridium septicum, Peptostreptococcus sp. and Parabacteroides distasonis have been reported as gas-forming anaerobic bacteria,5-7 there are a few reports on gas-producing lesions caused by B. fragilis,8-10 only one case of gas-forming VGI due to B. fragilis, which was isolated only from blood cultures, has been reported.10 To the best of our knowledge, there are no reports of gas-forming abscesses associated with PVGI caused by B. fragilis identified from the drainage fluid and blood cultures.
PVGI is characterized by high frequencies of polymicrobial infection (35 %) and negative culture (30 %).11 Approximately 70 % of B. fragilis bacteremia are caused by monomicrobial infection, 41 % of which are from the gastrointestinal tract and 29 % are unknown.12 In our case, B. fragilis was detected in the blood cultures before the administration of empirical antibiotics. Later when gas was found in the aneurysm, Gram staining of the drainage fluid showed Gram negative rods, 16S rRNA gene sequencing of the fluid identified B. fragilis, but the culture of the fluid was negative. Although the sensitivity of 16S rRNA sequencing for culture negative specimens is generally low, specimens with a positive Gram staining test had a markedly higher 16S rRNA sequencing-positive rate than -negative rate (44.3 % and 4.3 %, respectively; p < 0.001).13 Therefore, it may be useful to perform gene sequencing to identify a causative bacterium in clinical specimens when key culture tests are negative, but a Gram stain is positive. From these findings, we diagnosed this case as monomicrobial PVGI caused by B. fragilis. During the emergency open surgery for aortic replacement at admission, our patient had findings suspicious of mycotic aneurysm, but no culture on the surgical specimen was performed. It is possible that the patient had primarily an infected aneurysm caused by B. fragilis, resulting in PVGI and bacteremia.
In the treatment of infections requiring long-term antibiotic therapy, such as PVGI, gene sequencing possibly has greater contribution in shortening the duration of broad-spectrum antibiotic treatment, reducing the risk of patient morbidity due to infection with drug-resistant organisms and Clostridioides difficile, and reducing the economic burden of prolonged hospitalization and continuous use of expensive broad-spectrum antibiotics. However, when conducting gene sequencing, it should be noted that the organism detected may not be the only causative organism, or may not be the responsible bacterium at all. It is necessary to consider the possibility of contamination, polymicrobial infection, and infection in other sites based on cultures, imaging findings, and the clinical course.14,15
ConclusionWe reported a case of PVGI, in which drainage fluid culture collected under antibiotic treatment was negative, but 16 s rRNA sequencing identified the causative organism to be B. fragilis, facilitating the choice of an appropriate antibiotic regimen for the infection. In diseases requiring long-term antibiotic therapy, identification of the causative organism by gene sequencing is useful to decide appropriate antibiotic therapy.
Consent for publicationInformed consent was obtained from the patient for publication of this case report and accompanying images.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.