Patients infected with SARS-CoV-2 can develop acute kidney injury (AKI), associated with adverse clinical outcomes. In Mexico, an AKI incidence of 60.7% was reported in patients with COVID-19. Serum cystatin C is a well-known marker for AKI. It has been postulated as a marker for mortality in Chinese patients with COVID-19. Information regarding levels of cystatin C in COVID-19-infected patients is nonexistent among Mexican or Latin American populations.
AimThis work aimed to assess the level of cystatin C as an indicator of AKI and mortality among COVID-19 patients from Mexico.
MethodsA cross-sectional study among 38 adults was performed in the Regional High Specialty Hospital of the Yucatan Peninsula in Merida, Yucatan, Mexico. Baseline characteristics and clinical and biomechanical parameters were collected, and serum levels of cystatin C were measured by ELISA.
ResultsA total of 71% (27 patients) with COVID-19 developed AKI; 78% were men, and 22% were women. In addition, 60% of individuals (16 men; 7 women) died due to COVID-19 complications. Serum levels of cystatin C were higher in those individuals who developed AKI (p = 0.001). A logistic regression model indicated that individuals with serum levels of cystatin C above 0.84 ng/mL had a 23-fold increased risk of developing AKI (OR, 23.7, 95% CI, 2.59-217.00, p = 0.005). However, increased cystatin C was not independently associated with mortality in the Mexican population (HR, 1.01, 95% CI, 0.66-1.56, p = 0.959).
ConclusionThe results suggest that serum levels of cystatin C indicate AKI in COVID-19 patients. Although we recommend caution when using serum cystatin C levels as an indicator of mortality among the Mexican population, it is essential to note that cystatin C elevates earlier than creatinine, which is an advantage for timely clinical interventions.
The year 2020 was plagued by the coronavirus disease 2019 (COVID-19) that caused severe acute respiratory syndrome (SARS-CoV-2).1 Since then, multiorgan dysfunction has been observed in patients with COVID-19, and renal damage has been reported to be a common complication.2 From the first descriptive studies, it was documented that hospitalized patients infected by SARS-CoV-2 could develop acute kidney injury (AKI), which is associated with higher mortality rates;3,4 however, changes in the prevalence among the population have been reported.4-6 Mexico is one of the countries most affected by COVID-19 worldwide, with the number of deaths (253,155 per 100,000 population) peaking in August 2021.7 Moreover, an AKI incidence of 60.7% in Mexican patients has been reported.8 This rate can be exacerbated by overactive activation of inflammation caused by a high prevalence of obesity, diabetes, and hypertension in the Mexican population.9,10
Serum cystatin C is a highly sensitive and specific inflammatory marker of kidney function.11 Some reports from China support the usefulness of cystatin C as a predictive marker for AKI and mortality in COVID-19 patients.12 Nevertheless, of cystatin C is not broadly performed in patients with COVID-19 in clinical practice, and the vast majority of studies were conducted in China. Information regarding the levels of cystatin C in COVID-19-infected patients is nonexistent in Mexican or Latin American populations. Hence, this study hypothesized that the levels of cystatin C could be used as an indicator of kidney dysfunction, and similar to other studies, as a useful marker for mortality in COVID-19 cases. In this sense, this work aimed to determine the levels of cystatin C and assess its usefulness as an indicator of AKI and mortality among COVID-19 patients from Mexico.
Materials and methodsStudy population and designA cross-sectional study was performed on individuals who attended the COVID specialty unit at the Regional High Specialty Hospital (HRAEPY in the Spanish acronym) in Merida, Yucatan, between June and December 2020. The sample size calculation used a simple random sampling formula using a 95% confidence interval and an estimation error of 15%. A total of 616 patients were hospitalized in the COVID unit during the study period. Based on the calculation, the minimum required sample size would be 38 patients.
Thirty-eight hospitalized patients (28 men and 10 women) admitted to the COVID unit with SARS-CoV-2 infection confirmed by polymerase chain reaction, aged between 25 and 79 years and without a report of AKI were selected for this study. In our hospital, patients with COVID-19 are classified into severe or critical stages (patients who require mechanical ventilation, sedation, and prolonged bed rest).13 Creatinine levels of hospitalized patients were monitored every day, and those subjects who showed an increase of ≥ 0.3 mg/dL or ≥ 1.5 times baseline were considered as patients who developed AKI and were included in the study. Patients under 18 years of age and those with end-stage kidney disease or with a kidney transplant were excluded from this study. This research project was approved by the hospital Ethics Committee (No. CONBIOETICA-31-CEI-002-20170731) with the identification code 2020-021. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) was used to assess the methodological quality of this work.
Clinical characteristics, biochemical parameters, and cytokine assayFollowing a standard protocol, baseline and clinical characteristics data including symptoms, comorbidities, and intravenous administration of nephrotoxic drugs such as norepinephrine, midazolam, and propofol, were collected. Blood samples for biochemical parameters were collected during the 72 hours after individuals were admitted into the COVID unit from the hospital. Biochemical parameters were determined using validated methods (autoanalyzer COBAS® Integra 400 Plus, Roche Diagnostics). Serum was obtained by centrifugation of collection tubes at 3500 rpm for 10 min and stored at -20°C until use. Serum levels of cystatin C were determined in duplicate by an enzyme-linked immunosorbent assay (ELISA) according to the instructions of the manufacturers (Human Cystatin C Platinum ELISA kit #BMS2279, Bender MedSystems, Vienna, Austria). Raw data were analyzed with GraphPad Prism software. Patients’ survival status (dead or alive) was collected 90 days after hospital admission.
AKI classificationAKI was defined by the KDIGO criteria (Kidney Disease Improving Global Outcomes) as an increase in serum creatinine (sCr) by ≥ 0.3 mg/dL (≥ 26.5 mmol/L) within 48 hours or ≥ 1.5 times baseline, known or presumed to have occurred within the prior seven days. Furthermore, AKI was classified into three stages according to the sCr: a) Stage 1 increase in sCr level by ≥ 0.3 mg/dL in 48 hours or an increase of 1.5- to 1.9-fold change; b) Stage 2 increase in sCr from 2 to 2.9 times baseline; and c) Stage 3, increase sCr 3 times baseline or ≥ 4.0 mg/dL (≥ 353.6 mmol/L).14 Because only one patient developed stage 2 AKI, individuals were classified into AKI and without AKI groups for further statistical analyses.
Statistics analysesStatistical analyses were performed using the statistical package Jamovi (Version 1.2). Patients were assigned to either no AKI or AKI groups based on AKI development. Analysis was carried out on the complete sample (men = 28, women = 10). For categorical variables, absolute and relative frequencies by AKI status are shown. Continuous variables were tested by the Shapiro–Wilk test (p < 0.05) to assess if the variable was normally distributed. Continuous variables between groups were compared using Student's t test or the Mann–Whitney U test. The cutoff value of cystatin C was based on the Youden Index (sensitivity + specificity −1).15 Furthermore, logistic regression was performed to evaluate the risk of developing AKI calculated by the odds ratio (OR). Mortality risk was assessed with a survival analysis calculated by the hazard ratio (HR). For all analyses, a p < 0.05 was considered significant. No missing data or missing values were found.
ResultsA total of 27 (71%) patients with COVID-19 developed AKI (stage 1, n = 7; stage 2, n = 1, stage 3, n = 19); 21 (78%) were men, and 6 (22%) were women. In addition, 42% (n = 16) of men and 18% (n = 7) of women died due to COVID-19 complications. From the group that did not survive, four individuals were classified at stage 1, one at stage 2, sixteen at stage 3. Two did not develop AKI. The mean age of the AKI group was higher (60.2 ± 10.2 years) than that of the group without AKI (52.5 ± 14.9 years). Nonetheless, there were no significant differences (p = 0.073). COVID-19 symptoms were more frequent among the patients with AKI; shortness of breath was significantly more recurrent (p = 0.03) in the AKI group. Diabetes, hypertension, and obesity were the most common comorbidities among patients with AKI. Notably, all individuals needed oxygen support, and 28 (73%) patients needed ventilator support (Table 1).
Baseline characteristics of COVID-19 patients classified by AKI development.
SD, standard deviation; AKI, acute kidney injury.
The clinical parameters of COVID-19 patients were compared among groups (without AKI vs. AKI). The respiratory rate (p = 0.01) and oxygen saturation (p = 0.002) were found to be significantly higher in the individuals who developed AKI. Additionally, higher serum levels of ferritin (p = 0.03), creatinine (p = 0.03), and urea (p = 0.02) were reported in COVID-19 patients who developed AKI. In addition, serum levels of cystatin C (p = 0.001) were identified to be higher in individuals infected by SARS-CoV-2 who developed AKI (Table 2). Moreover, the same clinical and biomechanical parameters were evaluated among patients according to their survival status (dead vs. alive), and only the serum level of urea (p = 0.02) was found to be significantly different between groups (Table 3).
Clinical and biochemical parameters of COVID-19 patients classified by AKI.
SD, standard deviation; AKI, acute kidney injury; ALT, alanine aminotransferase; AST, aspartate transaminase; CRP, C-reactive protein.
Clinical and serum biochemical parameters of COVID-19 patients classified by survival status.
Dependent variable: Dead (yes = 1, no = 0), SD: standard deviation. ALT, alanine aminotransferase; AST, aspartate transaminase; CRP, C-reactive protein.
Serum levels of cystatin C were higher in the group of individuals who developed AKI. Cystatin C levels were significantly elevated in individuals with COVID-19 and AKI (1.39 ± 0.88 ng/mL) compared with individuals who did not develop AKI (0.73 ± 0.14 ng/mL) (Table 4).
Cystatin C levels in COVID-19 patients classified by AKI.
| Cytokine | Without AKI (n=12) | AKI (n=27) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | 25% | Median | 75% | Max | Mean | SD | Min | 25% | Median | 75% | Max | |
| Cystatin C (mg/dL) | 0.73 | 0.14 | 0.46 | 0.64 | 0.76 | 0.80 | 1.00 | 1.39 | 0.88 | 0.37 | 0.73 | 1.17 | 1.74 | 3.71 |
AKI, acute kidney injury; SD, standard deviation; Min, minimum; Max, maximum.
A logistic regression model indicated that the OR estimates of AKI were associated with serum levels of cystatin C (OR, 20, 95% CI, 2.203-181.55, p = 0.049). Additionally, a cutoff value of 0.84 ng/mL had sensitivity of 70.4% and specificity of 90.9% with a Youden Index of 0.61 (OR, 23.75, 95% CI, 2.59-217.65, p = 0.005). This suggests that individuals with serum levels of cystatin C above 0.84 ng/mL have 23-fold higher risk of developing AKI. After adjusting for age, diabetes, and hypertension, serum levels of cystatin C remained independently associated with AKI development (Table 5).
Risk of developing AKI among COVID-19 patients adjusted for age, diabetes and hypertension.
OR, odds ratio; AOR, Adjusted odds ratio.
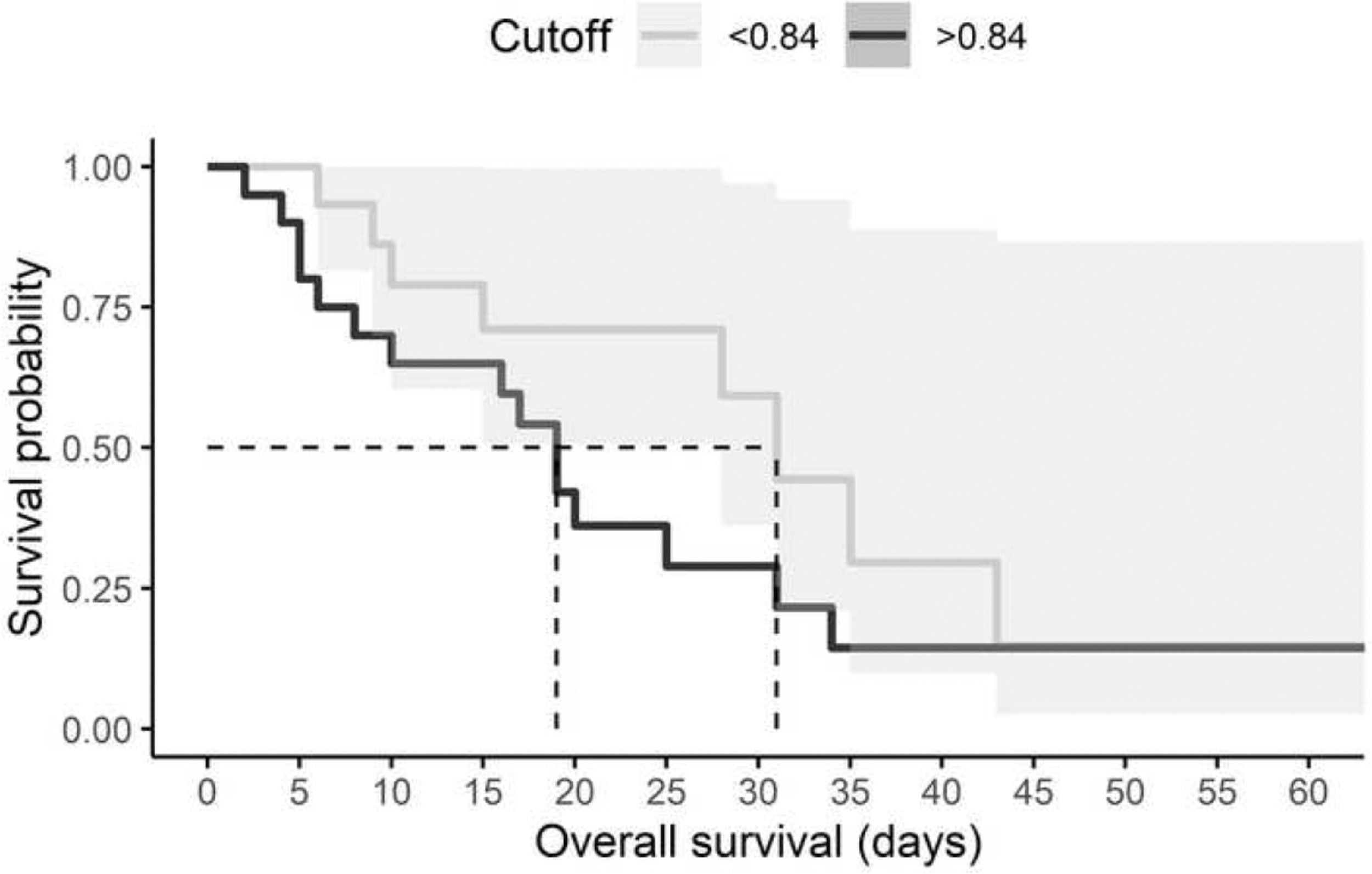
Furthermore, the risk of death in relation to cystatin C level was estimated using survival analysis. All individuals with a diagnosis of AKI and a high level of cystatin C died within 19 days, although the hazard ratio (HR) was not significant (HR: 1.01, 95% CI, 0.66-1.56, p = 0.959) (Fig. 1). Mechanical ventilation was the variable that significantly impacted mortality (HR: 8.85 95% CI, 1.19-65.83, p = 0.03) (Table 6).
The mortality risk among COVID-19 patients.
HR, hazard ratio.
Our study showed a high incidence of AKI (71%) and mortality (60.52%). New scientific evidence regarding the usefulness of serum cystatin C levels and the risk of developing AKI and mortality in patients infected by SARS-CoV-2 in Mexico was presented in this study. Of note, cystatin C serum levels predicted kidney dysfunction but not mortality among the Mexican population. Only two studies in Mexico have described the occurrence of AKI in individuals infected by SARS-CoV-2, and the results showed discrepancies in the frequencies 34% - 58.6%.16,17 Our results showed a higher frequency of patients with COVID-19 who developed AKI (71%), and 70% were classified as stage 3. This result is in accordance with Casas-Aparicio et al., who reported that half of the individuals who developed AKI were at stage 3.17 One reason that could explain the increased occurrence of AKI in our study population is the highest frequency of obesity and diabetes in the population from Yucatan,18 which are associated with poor prognosis in patients with COVID-19. Our study showed that patients who developed AKI had higher frequencies of comorbidities, such as diabetes, hypertension, and/or obesity, which is in line with previous studies.19 Some risk factors for AKI and its association with mortality have been reported in the general population and Mexican patients with COVID-19.17 The mortality rate was found to be high in this study (60.5%), and most of them were at stage 3 (56.5%). In fact, 21 out of 23 patients who died had renal dysfunction.
It has been reported that cystatin C is an inflammatory biomarker associated with renal function.20 Indeed, cystatin C has been proposed as an endogenous serum marker for the early assessment of variations in the glomerular filtration rate (GFR), which is even more sensitive than creatinine.21 Only a few studies have reported the clinical impact of serum cystatin C in patients infected by SARS-CoV-2. However, the available data are based on patients from China.12 Yang et al. reported that using a cystatin C cutoff of > 0.93 mg/dL was associated with all-cause death (OR = 5.585, 95% CI: 2.328–13.397).22 Furthermore, Li Yan et al. described cystatin C as an independent risk factor associated with death in patients with COVID-19.23 Nevertheless, Yannan et al. found that a reduction in the GFR calculated by serum creatinine (eGFRcr) showed a significant association with death. However, eGFR-cystatin C is a poor predictive factor for mortality.24 A recently published meta-analysis concluded that higher concentrations of serum cystatin C were associated with mortality in COVID-19 patients. However, the 13 included studies were conducted in China, and extreme heterogeneity between studies was observed (I2 = 97.5%, p<0.001).12
Although in our study, patients who developed AKI had elevated levels of CRP and ferritin, which suggests a severe inflammatory condition, cystatin C serum levels did not show clinical significance in the prognosis of mortality. This discrepancy could be due to the small study sample, as well as the delay in patients seeking admission to health services and medical treatment25 in our hospital. Therefore, most of the patients had poor health conditions at the time of hospital admission.
In addition, another risk factor was the requirement for mechanical ventilation, which was significantly associated with the development of AKI and survival status. Some of the major causes of AKI are systemic hypoxia, ischemia, and nephrotoxicity. It has been described that kidney damage can activate the inflammatory processes.2 Hyper-inflammation has been associated with COVID-19,19 mainly observed in the high levels of expression of interleukin 6 (IL-6).26,27
Some limitations of this study included the limited numbers of enrolled patients from a single hospital center; hence, our results need further confirmation by evaluating larger groups from different centers in the country. Another limitation is the study design; therefore, the results should be interpreted as merely an association and no causality. Nevertheless, the data presented represent the first evidence of the clinical impact of cystatin C in hospitalized patients with COVID-19 in Mexico and Latin America.
ConclusionTogether, we reported a strong association between serum levels of cystatin C and the development of AKI in patients with COVID-19. A cutoff of 0.84 ng/mL was established for our population. This work suggests that serum level of cystatin C is an indicator of AKI in COVID-19 patients. Although we recommend caution when using serum cystatin C levels as a predictor for mortality in the Mexican population, it is important to note that cystatin C elevates earlier than creatinine, which is an advantage for timely clinical interventions.
Authors' contributionsConception and design: AL Gutiérrez-Solis; Administrative support: K Ramos-Santos; Provision of study materials or patients: A Cortes-Telles, MA Uc-Miam, A Ávila-Nava and Lugo Roberto; Collection and assembly of data: AL Gutiérrez-Solis, K Ramos-Santos, R Chim Aké; Data analysis and interpretation: AL Gutiérrez-Solis and K Ramos-Santos; Manuscript writing: All authors; Final approval of manuscript: All authors.
FundingThis research received no external funding and the Cystatin C kit was funded by Hospital Regional de Alta Especialidad de la Península de Yucatán.
Ethics approval and consent to participateThis study was part of a project titled “Determination of serum markers NGAL, CysC, TIMP-2 and IGFBP7 and their relationship with acute kidney injury in patients with COVID-19” (No. 2020-021) that has been approved by Research Committee and the Ethics Committee from the Regional High Speciality Hospital of the Yucatan Peninsula (No. CONBIOETICA-31-CEI-002-20170731).
Consent of publicationAuthors agree to allow the publication and declare that the submitted work is not presented and will not be published elsewhere in whatever language and published article will not be shared with anyone without earlier written permission of the publisher, except for academic purposes. Also, the authors agree to allow the publication and distribution of the materials submitted in all available forms, without limiting territory or language, provided that the material is accepted for publication. Authors confirm that all information is original and free from plagiarism.
The authors are very grateful to the participants. Also, we would like to thank Mr. Julio Vega for helping in data analysis.
Availability of data and material: Data underlying this work is available upon reasonable request. Requests for data should be addressed to the corresponding author.